Abstract
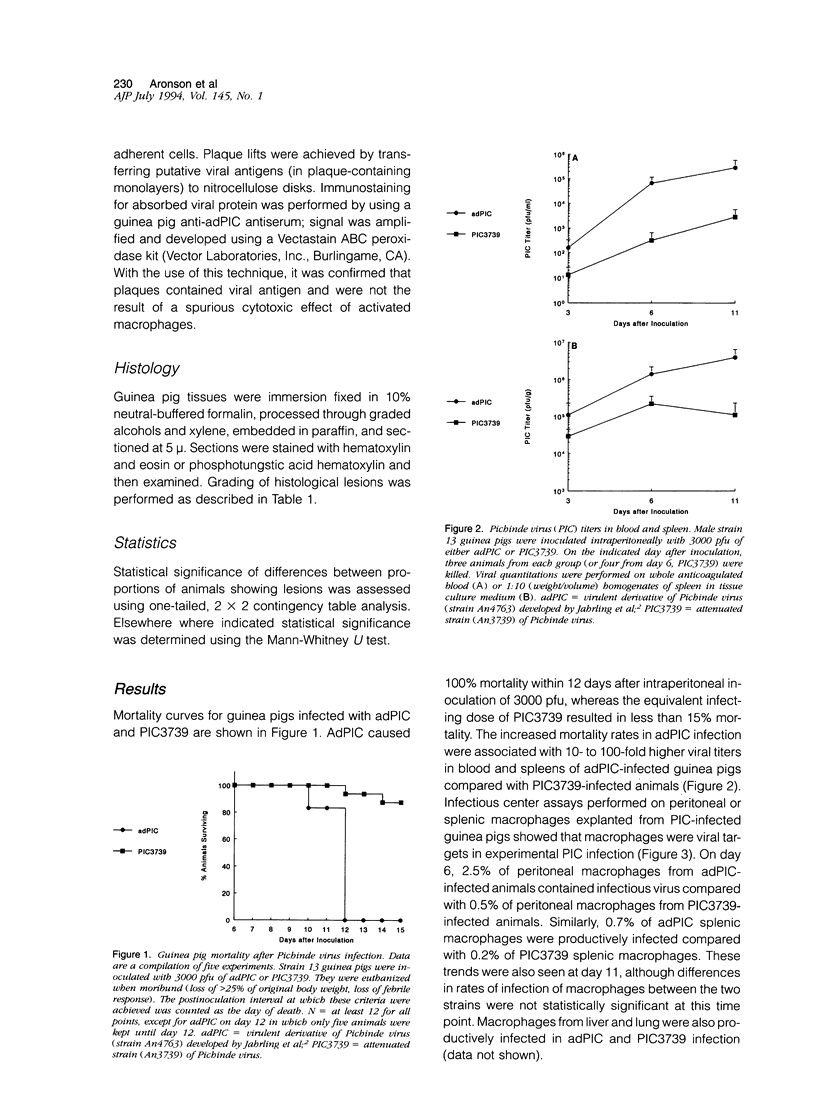
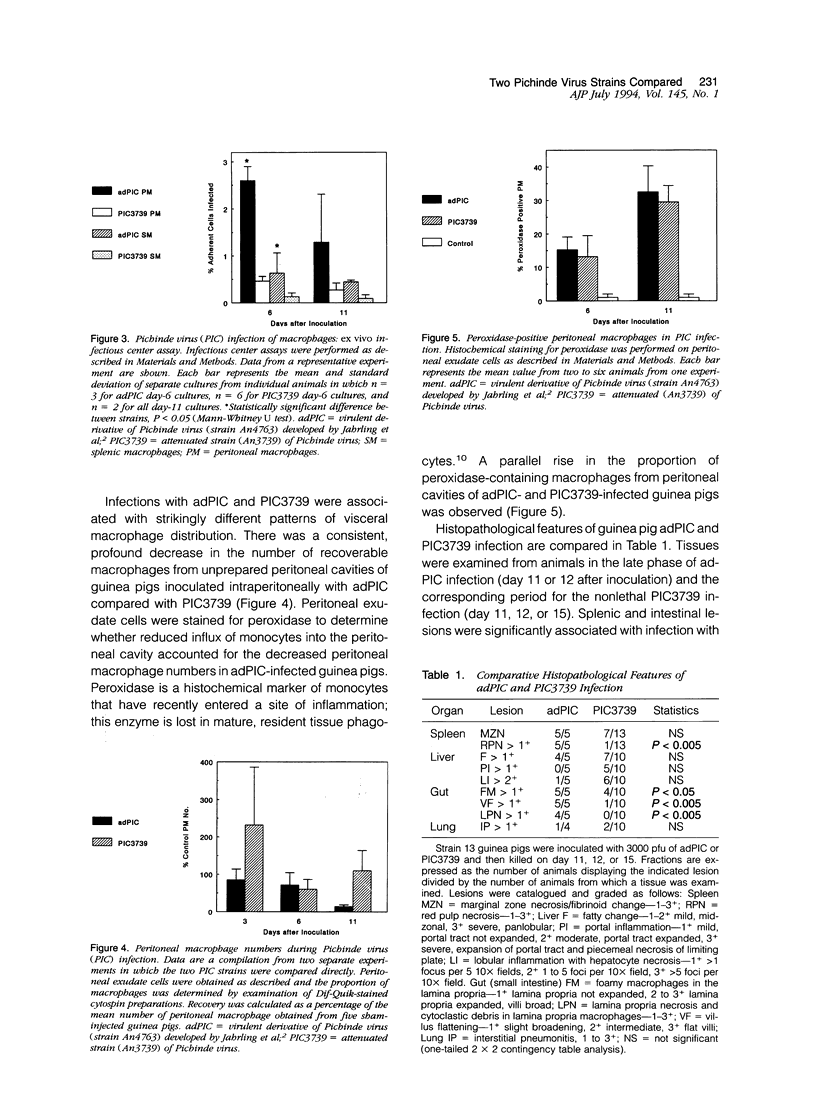
A guinea pig passage-adapted strain of the arena-virus Pichinde (adPIC) is highly virulent in inbred guinea pigs, whereas the related strain PIC3739 is attenuated. Both viruses were macrophage tropic and infected peritoneal, splenic, liver, and alveolar macrophages during experimental Pichinde virus infection. Infection with the virulent strain was associated with unlimited viral replication in the face of exaggerated delayed-type hypersensitivity response, manifested by the macrophage disappearance reaction. Histopathological lesions unique to adPIC-infected guinea pigs included intestinal villus blunting with mucosal infiltration by pyknotic debris-laden macrophages and apoptosis of crypt epithelial cells. Splenic red pulp necrosis was also significantly associated with adPIC infection but not PIC3739 infection. These findings may provide clues to the pathogenesis of a group of poorly understood human viral hemorrhagic fevers.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D., Dimock K., Cash P., Rawls W. E., Leung W. C., Bishop D. H. Analyses of the genomes of prototype pichinde arenavirus and a virulent derivative of Pichinde Munchique: evidence for sequence conservation at the 3' termini of their viral RNA species. Virology. 1982 Jan 15;116(1):363–367. doi: 10.1016/0042-6822(82)90429-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Rawls W. E. Variation between strains of hamsters in the lethality of Pichinde virus infections. Infect Immun. 1977 May;16(2):413–421. doi: 10.1128/iai.16.2.413-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. M., Jenson A. B., Peters C. J., Geyer S. J., Barth J. F., McPherson R. A. Pathogenesis of Pichinde virus infection in strain 13 guinea pigs: an immunocytochemical, virologic, and clinical chemistry study. Am J Trop Med Hyg. 1993 Jul;49(1):10–24. doi: 10.4269/ajtmh.1993.49.10. [DOI] [PubMed] [Google Scholar]
- Cosgriff T. M. Viruses and hemostasis. Rev Infect Dis. 1989 May-Jun;11 (Suppl 4):S672–S688. doi: 10.1093/clinids/11.supplement_4.s672. [DOI] [PubMed] [Google Scholar]
- D'Silva H., Yoshida T., Cohen S. The effect of lymphokines on macrophage accumulation and disappearance in the peritoneal cavity. J Exp Pathol. 1983;1(1):61–69. [PubMed] [Google Scholar]
- Friedlander A. M., Jahrling P. B., Merrill P., Tobery S. Inhibition of mouse peritoneal macrophage DNA synthesis by infection with the arenavirus Pichinde. Infect Immun. 1984 Jan;43(1):283–288. doi: 10.1128/iai.43.1.283-288.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelkin L., Jahrling P. B. Virus-initiated septic shock. Acute death of Venezuelan encephalitis virus-infected hamsters. Lab Invest. 1975 Jan;32(1):78–85. [PubMed] [Google Scholar]
- Jahrling P. B., Frame J. D., Smith S. B., Monson M. H. Endemic Lassa fever in Liberia. III. Characterization of Lassa virus isolates. Trans R Soc Trop Med Hyg. 1985;79(3):374–379. doi: 10.1016/0035-9203(85)90386-4. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Hesse R. A., Rhoderick J. B., Elwell M. A., Moe J. B. Pathogenesis of a pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect Immun. 1981 May;32(2):872–880. doi: 10.1128/iai.32.2.872-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., McCormick J. B., Webb P. A., Smith E. S., Elliott L. H., King I. J. Clinical virology of Lassa fever in hospitalized patients. J Infect Dis. 1987 Mar;155(3):456–464. doi: 10.1093/infdis/155.3.456. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Lucia H. L., Coppenhaver D. H., Harrison R. L., Baron S. The effect of an arenavirus infection on liver morphology and function. Am J Trop Med Hyg. 1990 Jul;43(1):93–98. doi: 10.4269/ajtmh.1990.43.93. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., Walker D. H., King I. J., Webb P. A., Elliott L. H., Whitfield S. G., Johnson K. M. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am J Trop Med Hyg. 1986 Mar;35(2):401–407. doi: 10.4269/ajtmh.1986.35.401. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Buchmeier M. J., Rawls W. E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Invest. 1977 Nov;37(5):502–515. [PubMed] [Google Scholar]
- NELSON D. S., NORTH R. J. THE FATE OF PERITONEAL MACROPHAGES AFTER THE INJECTION OF ANTIGEN INTO GUINEA PIGS WITH DELAYED-TYPE HYPERSENSITIVITY. Lab Invest. 1965 Jan;14:89–101. [PubMed] [Google Scholar]
- Nestel F. P., Price K. S., Seemayer T. A., Lapp W. S. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992 Feb 1;175(2):405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. J., Liu C. T., Anderson G. W., Jr, Morrill J. C., Jahrling P. B. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev Infect Dis. 1989 May-Jun;11 (Suppl 4):S743–S749. doi: 10.1093/clinids/11.supplement_4.s743. [DOI] [PubMed] [Google Scholar]
- Polyak S. J., Rawls W. E., Harnish D. G. Characterization of Pichinde virus infection of cells of the monocytic lineage. J Virol. 1991 Jul;65(7):3575–3582. doi: 10.1128/jvi.65.7.3575-3582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Adoptive transfer of fluorescence-labeled cells shows that resident peritoneal macrophages are able to migrate into specialized lymphoid organs and inflammatory sites in the mouse. Eur J Immunol. 1990 Jun;20(6):1251–1258. doi: 10.1002/eji.1830200609. [DOI] [PubMed] [Google Scholar]
- Sarrat H., Camain R., Baum J., Robin Y. Diagnostic histopathologique des hépatites dues au virus Lassa. Bull Soc Pathol Exot Filiales. 1972 Sep-Oct;65(5):642–650. [PubMed] [Google Scholar]
- Sawicki W., Kucharczyk K., Szymanska K., Kujawa M. Lamina propria macrophages of intestine of the guinea pig. Possible role in phagocytosis of migrating cells. Gastroenterology. 1977 Dec;73(6):1340–1344. [PubMed] [Google Scholar]
- Sonozaki H., Cohen S. The macrophage disappearance reaction: mediation by a soluble lymphocyte-derived factor. Cell Immunol. 1971 Aug;2(4):341–352. doi: 10.1016/0008-8749(71)90069-4. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Iwata S., Uchida K., Fujiwara H. [The pathological study of enterosiderosis in guinea pigs]. Jikken Dobutsu. 1988 Apr;37(2):171–177. doi: 10.1538/expanim1978.37.2_171. [DOI] [PubMed] [Google Scholar]
- Trapido H., Sanmartín C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg. 1971 Jul;20(4):631–641. [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., McCormick J. B., Johnson K. M., Webb P. A., Komba-Kono G., Elliott L. H., Gardner J. J. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982 Jun;107(3):349–356. [PMC free article] [PubMed] [Google Scholar]
- Walker N. I., Bennett R. E., Axelsen R. A. Melanosis coli. A consequence of anthraquinone-induced apoptosis of colonic epithelial cells. Am J Pathol. 1988 Jun;131(3):465–476. [PMC free article] [PubMed] [Google Scholar]
- Winn W. C., Jr, Walker D. H. The pathology of human Lassa fever. Bull World Health Organ. 1975;52(4-6):535–545. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]




