Abstract
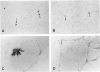
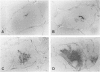
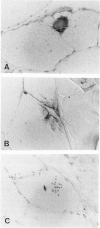
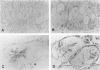
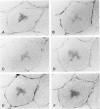
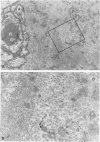
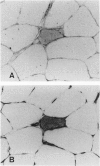
The microtubule-associated protein tau is a major cytoskeletal protein involved in the neurofibrillary tangles of Alzheimer's disease. Although tau is predominantly a neuronal protein, it has been demonstrated in glia and other nonneuronal cells. We describe the presence of microtubule-associated protein tau epitopes in various muscle fiber lesions in oculopharyngeal and Becker muscular dystrophy, dermatomyositis, central core disease, neurogenic atrophy, and in the recovery phase of an attack of malignant hyperthermia. Western blot demonstrated a 100- to 110-kd tau-immunoreactive protein probably corresponding to 'big tau' as described in peripheral nerves. Tau immunoreactivity in muscle fiber lesions usually co-localized with tubulin, although electron microscopy failed to show an increase in microtubules. Tau and tubulin reactivity also correlated with the presence of desmin and vimentin epitopes. Possible explanations for the presence of tau are briefly discussed.
Full text
PDF
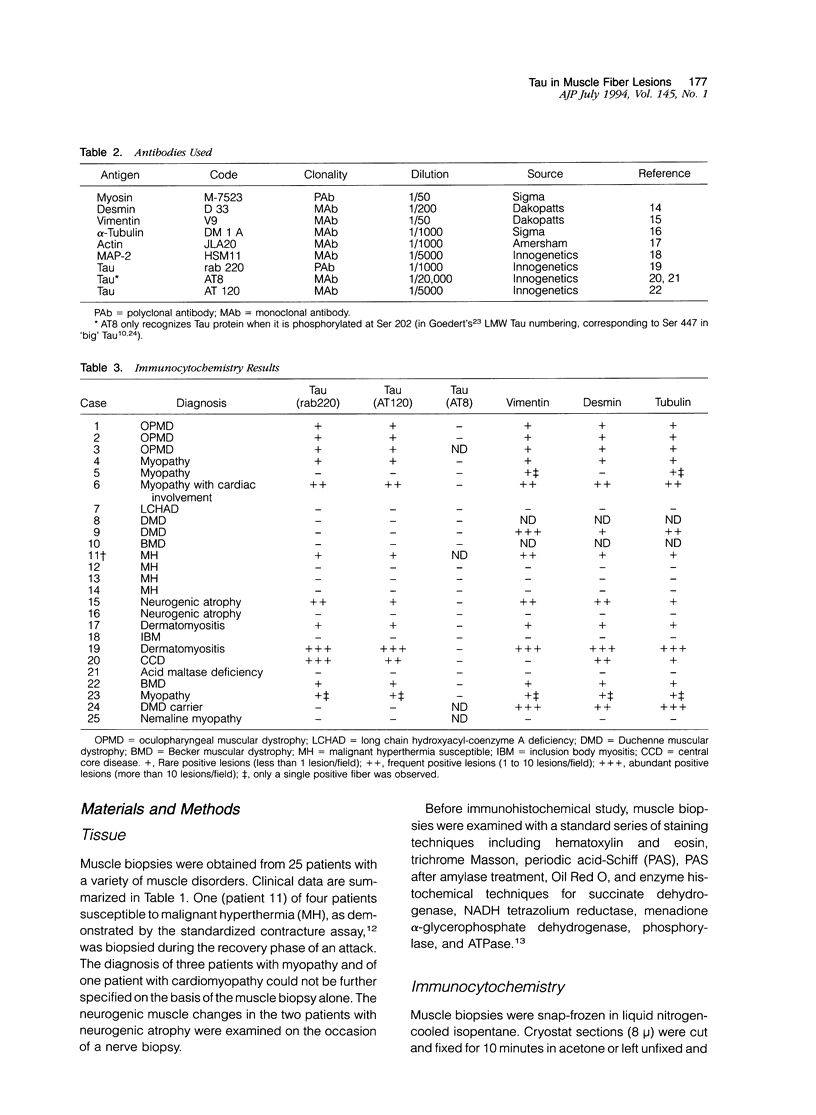
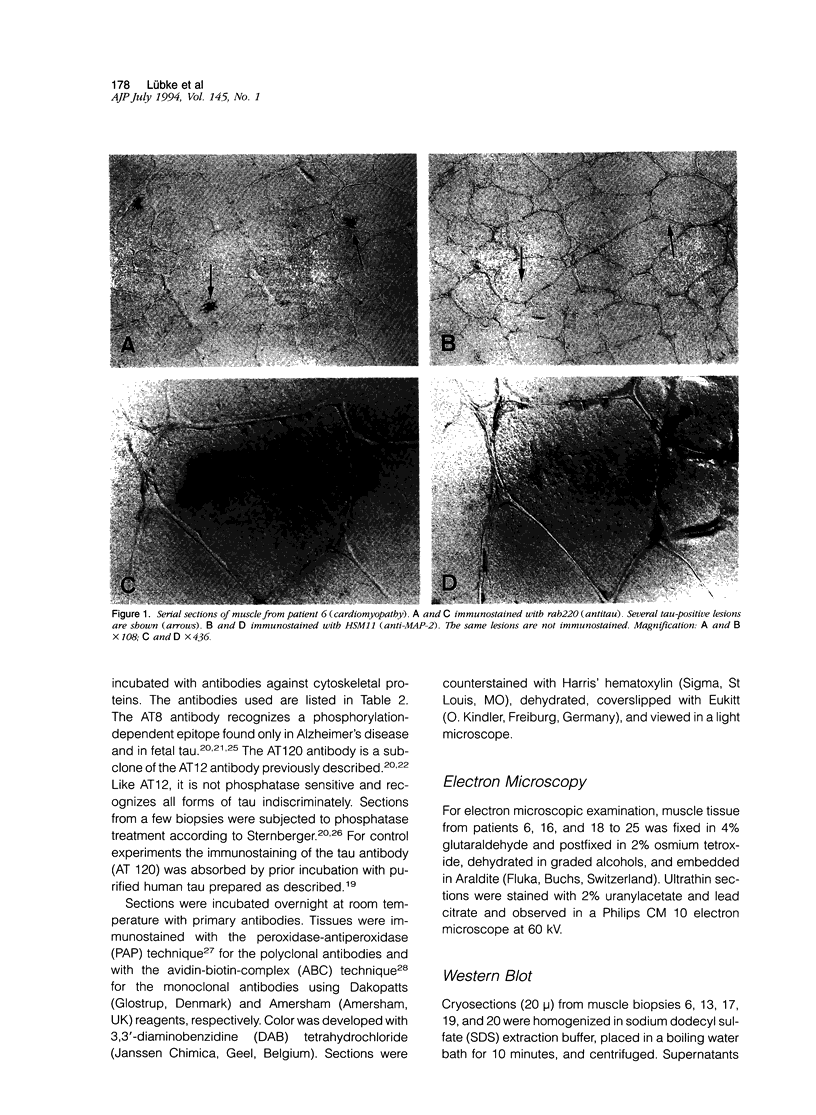
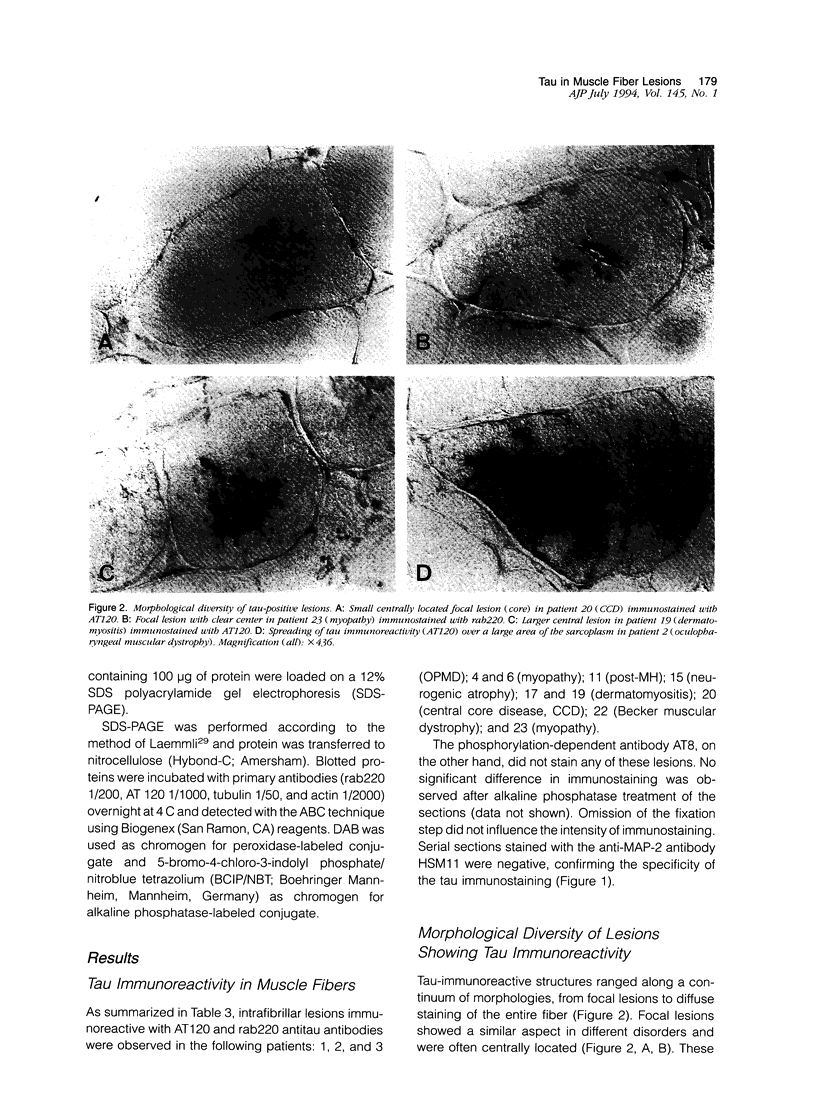
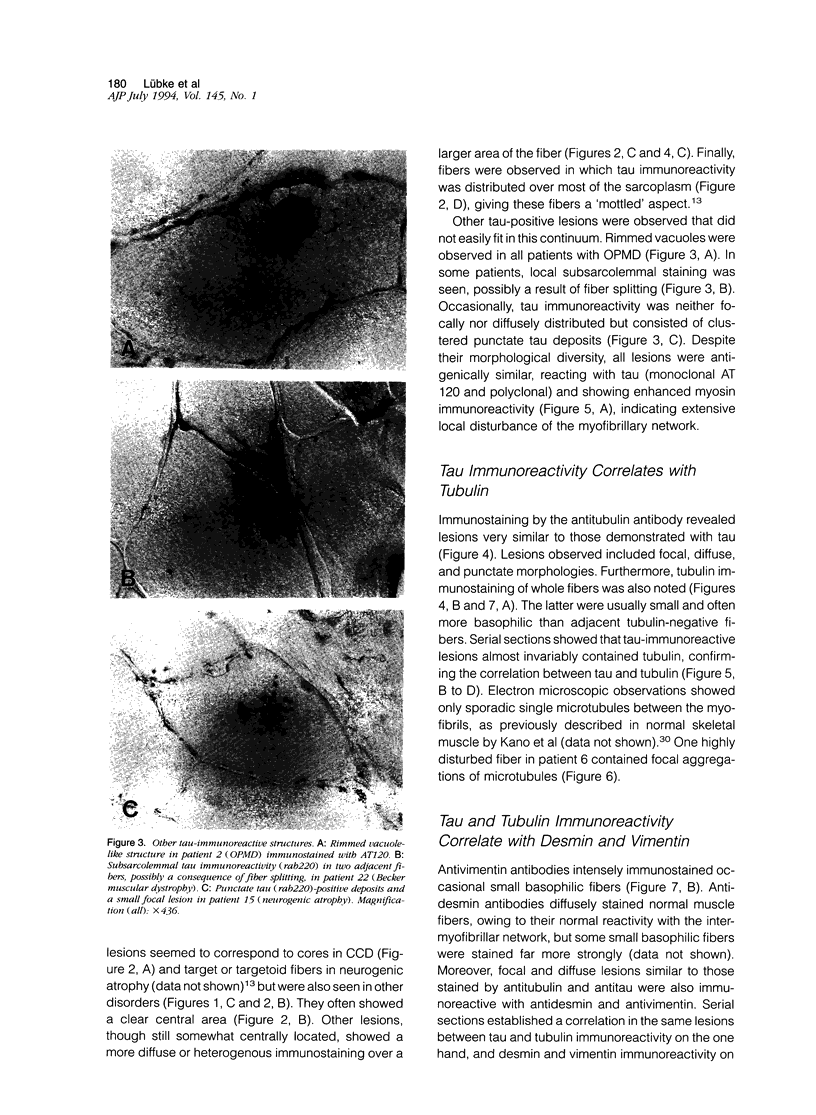

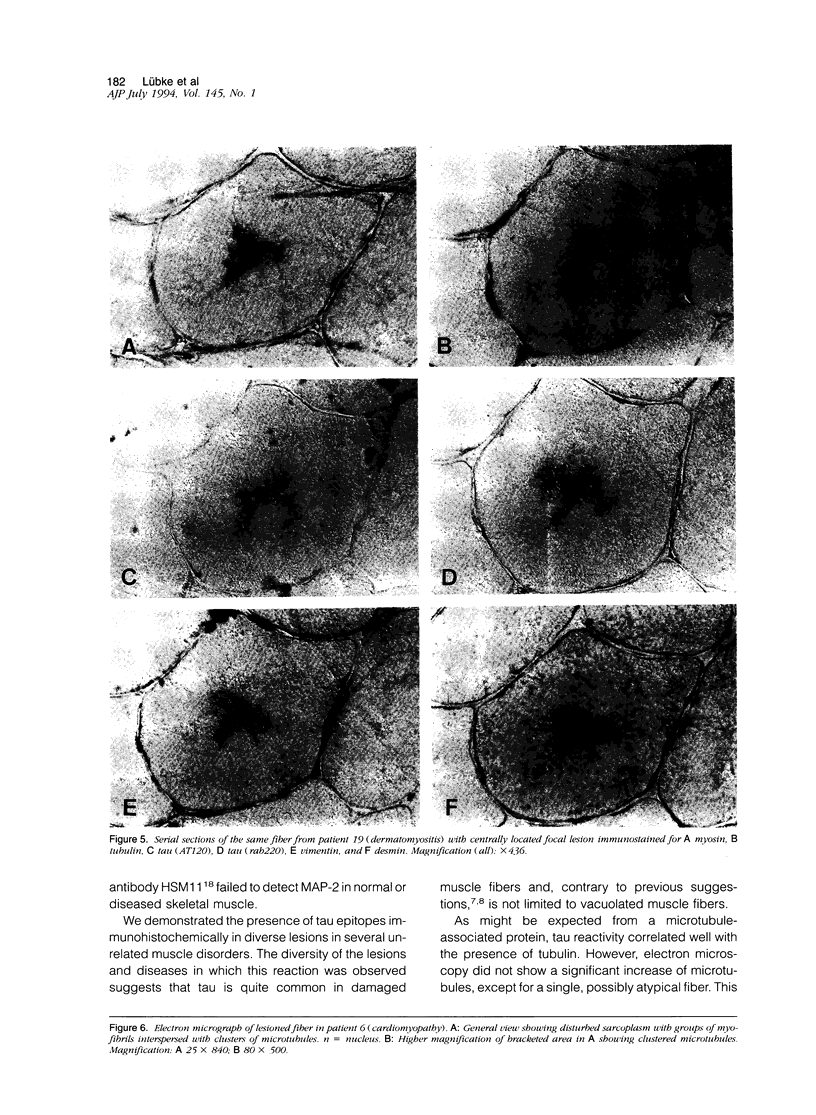
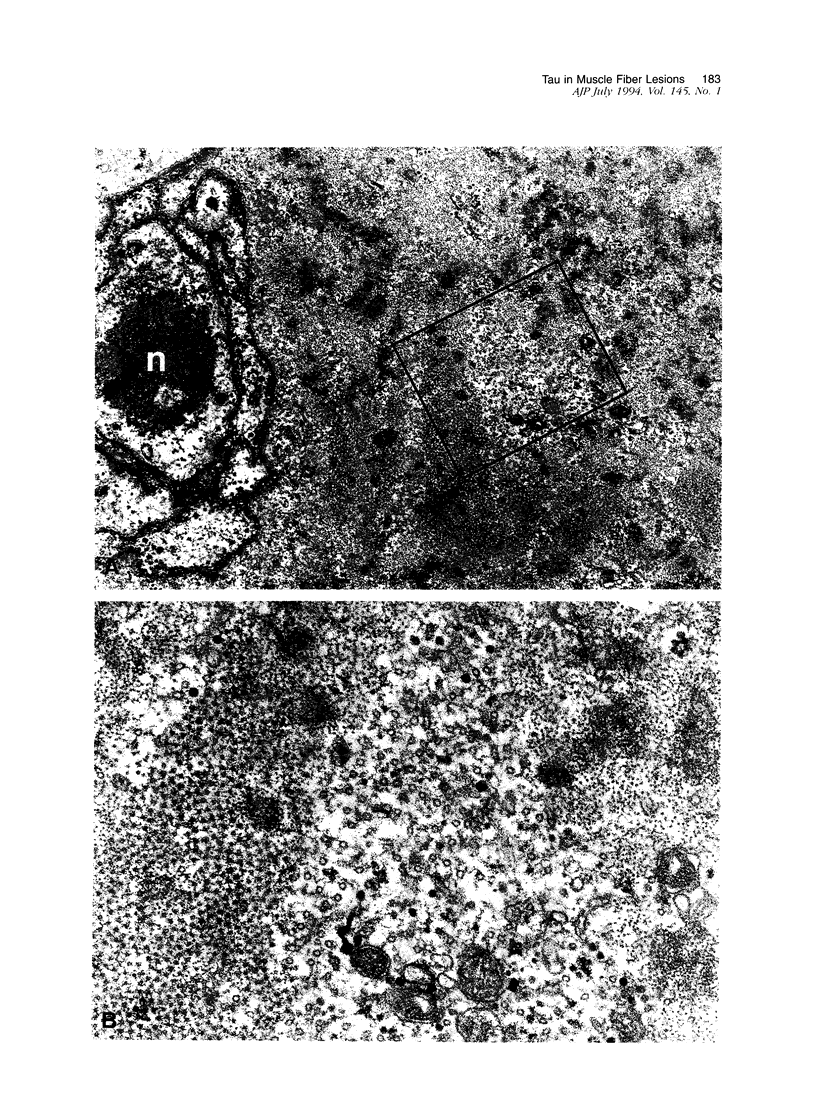
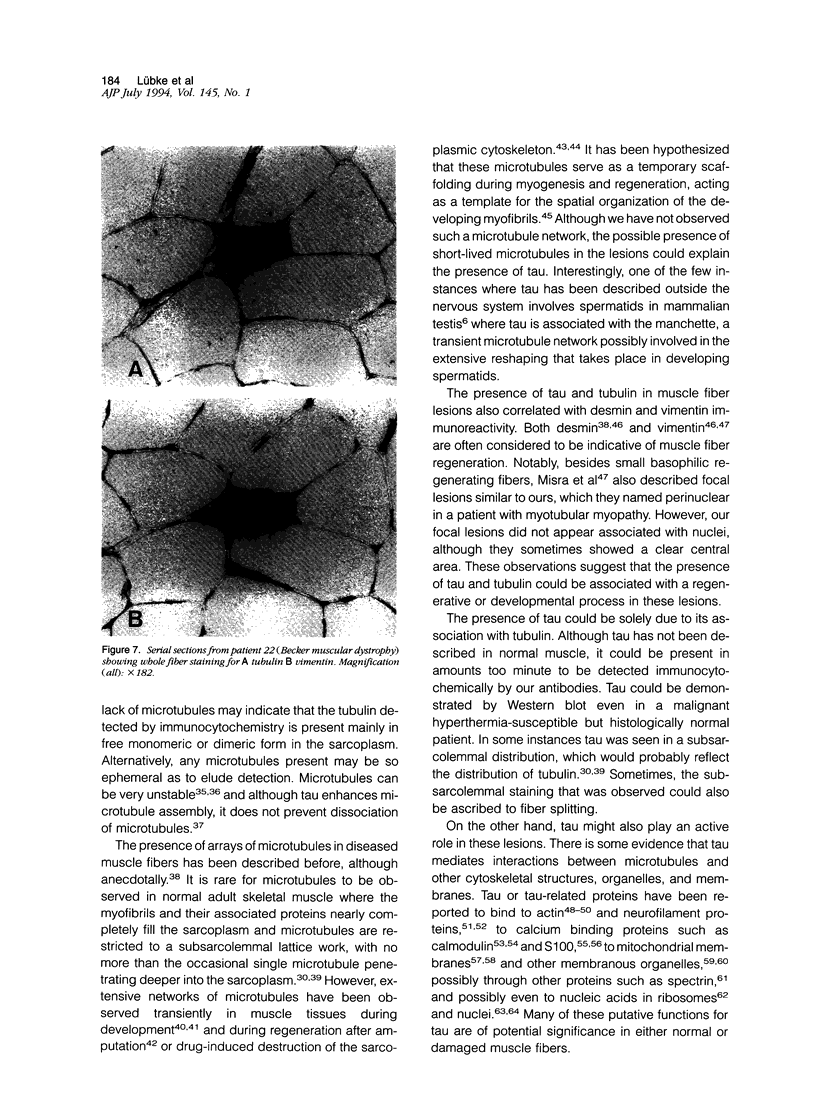
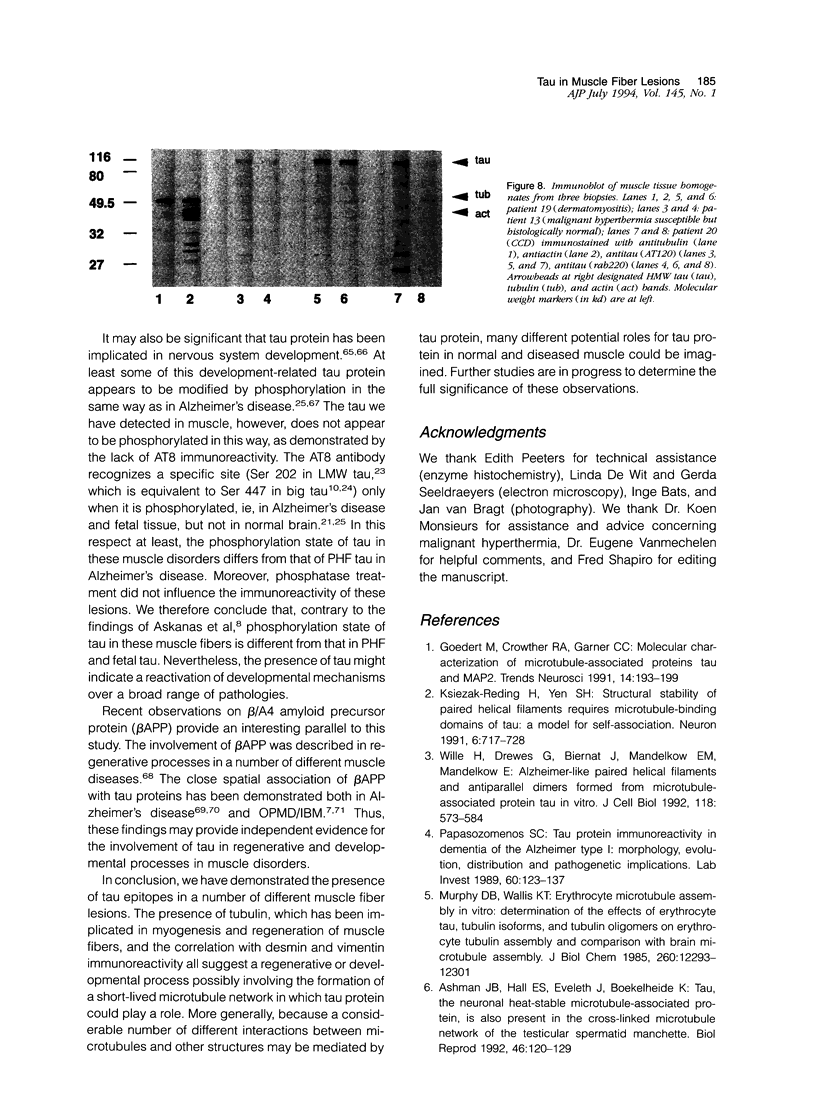



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan V. J., Kreis T. E. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986 Dec;103(6 Pt 1):2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antin P. B., Forry-Schaudies S., Friedman T. M., Tapscott S. J., Holtzer H. Taxol induces postmitotic myoblasts to assemble interdigitating microtubule-myosin arrays that exclude actin filaments. J Cell Biol. 1981 Aug;90(2):300–308. doi: 10.1083/jcb.90.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman J. B., Hall E. S., Eveleth J., Boekelheide K. Tau, the neuronal heat-stable microtubule-associated protein, is also present in the cross-linked microtubule network of the testicular spermatid manchette. Biol Reprod. 1992 Jan;46(1):120–129. doi: 10.1095/biolreprod46.1.120. [DOI] [PubMed] [Google Scholar]
- Askanas V., Alvarez R. B., Engel W. K. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993 Oct;34(4):551–560. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Bilak M., Alvarez R. B., Selkoe D. J. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994 Jan;144(1):177–187. [PMC free article] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Interactions between the microtubule-associated tau proteins and S100b regulate tau phosphorylation by the Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1988 Apr 25;263(12):5876–5883. [PubMed] [Google Scholar]
- Biernat J., Mandelkow E. M., Schröter C., Lichtenberg-Kraag B., Steiner B., Berling B., Meyer H., Mercken M., Vandermeeren A., Goedert M. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593–1597. doi: 10.1002/j.1460-2075.1992.tb05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H., Meltzer D. I., Feramisco J. R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984 Mar;98(3):847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudriau S., Vincent M., Côté C. H., Rogers P. A. Cytoskeletal structure of skeletal muscle: identification of an intricate exosarcomeric microtubule lattice in slow- and fast-twitch muscle fibers. J Histochem Cytochem. 1993 Jul;41(7):1013–1021. doi: 10.1177/41.7.8515044. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Simon C., Cassoly R., Pradel L. A. Interaction between microtubule-associated protein tau and spectrin. Biochimie. 1984 Apr;66(4):305–311. doi: 10.1016/0300-9084(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Correas I., Padilla R., Avila J. The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem J. 1990 Jul 1;269(1):61–64. doi: 10.1042/bj2690061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R., Giambanco I., Aisa M. C. Molecular interaction of S-100 proteins with microtubule proteins in vitro. J Neurochem. 1989 Aug;53(2):566–571. doi: 10.1111/j.1471-4159.1989.tb07371.x. [DOI] [PubMed] [Google Scholar]
- Flynn G., Joly J. C., Purich D. L. The 28,000 Mr microtubule-binding domain of microtubule-associated protein-2 also contains a neurofilament-binding site. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1453–1459. doi: 10.1016/s0006-291x(87)80295-4. [DOI] [PubMed] [Google Scholar]
- Gallanti A., Prelle A., Moggio M., Ciscato P., Checcarelli N., Sciacco M., Comini A., Scarlato G. Desmin and vimentin as markers of regeneration in muscle diseases. Acta Neuropathol. 1992;85(1):88–92. doi: 10.1007/BF00304637. [DOI] [PubMed] [Google Scholar]
- Georgieff I. S., Liem R. K., Couchie D., Mavilia C., Nunez J., Shelanski M. L. Expression of high molecular weight tau in the central and peripheral nervous systems. J Cell Sci. 1993 Jul;105(Pt 3):729–737. doi: 10.1242/jcs.105.3.729. [DOI] [PubMed] [Google Scholar]
- Georgieff I. S., Liem R. K., Mellado W., Nunez J., Shelanski M. L. High molecular weight tau: preferential localization in the peripheral nervous system. J Cell Sci. 1991 Sep;100(Pt 1):55–60. doi: 10.1242/jcs.100.1.55. [DOI] [PubMed] [Google Scholar]
- Goedert M., Crowther R. A., Garner C. C. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991 May;14(5):193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Crowther R. A. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1983–1987. doi: 10.1073/pnas.89.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. Evidence for actin filament-microtubule interaction mediated by microtubule-associated proteins. J Cell Biol. 1978 Sep;78(3):958–965. doi: 10.1083/jcb.78.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., George L., Tung Y. C., Kim K. S., Wisniewski H. M. Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2853–2857. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J Cell Biol. 1989 Nov;109(5):2275–2288. doi: 10.1083/jcb.109.5.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Forry-Schaudies S., Dlugosz A., Antin P., Dubyak G. Interactions between IFs, microtubules, and myofibrils in fibrogenic and myogenic cells. Ann N Y Acad Sci. 1985;455:106–125. doi: 10.1111/j.1749-6632.1985.tb50407.x. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jancsik V., Filliol D., Felter S., Rendon A. Binding of microtubule-associated proteins (MAPs) to rat brain mitochondria: a comparative study of the binding of MAP2, its microtubule-binding and projection domains, and tau proteins. Cell Motil Cytoskeleton. 1989;14(3):372–381. doi: 10.1002/cm.970140307. [DOI] [PubMed] [Google Scholar]
- Kanemaru K., Takio K., Miura R., Titani K., Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1992 May;58(5):1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- Kano Y., Fujimaki N., Ishikawa H. The distribution and arrangement of microtubules in mammalian skeletal muscle fibers. Cell Struct Funct. 1991 Jun;16(3):251–261. doi: 10.1247/csf.16.251. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Davies P., Yen S. H. Alz 50, a monoclonal antibody to Alzheimer's disease antigen, cross-reacts with tau proteins from bovine and normal human brain. J Biol Chem. 1988 Jun 15;263(17):7943–7947. [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Wolff J. Calmodulin binds to both microtubule-associated protein 2 and tau proteins. J Biol Chem. 1984 Jan 25;259(2):1226–1230. [PubMed] [Google Scholar]
- Lewis S. A., Wang D. H., Cowan N. J. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988 Nov 11;242(4880):936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Lin J. J. Mapping structural proteins of cultured cells by monoclonal antibodies. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):769–783. doi: 10.1101/sqb.1982.046.01.073. [DOI] [PubMed] [Google Scholar]
- Lindén M., Nelson B. D., Leterrier J. F. The specific binding of the microtubule-associated protein 2 (MAP2) to the outer membrane of rat brain mitochondria. Biochem J. 1989 Jul 1;261(1):167–173. doi: 10.1042/bj2610167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx K. A. High affinity DNA-microtubule associated protein interaction. Mol Cell Biochem. 1992 Jul 6;113(1):55–61. doi: 10.1007/BF00230885. [DOI] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J. J., Gheuens J. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Vanmechelen E., Van de Voorde A., Gheuens J. Affinity purification of human tau proteins and the construction of a sensitive sandwich enzyme-linked immunosorbent assay for human tau detection. J Neurochem. 1992 Feb;58(2):548–553. doi: 10.1111/j.1471-4159.1992.tb09754.x. [DOI] [PubMed] [Google Scholar]
- Miller M. W., al-Ghoul W. M., Murtaugh M. Expression of ALZ-50 immunoreactivity in the developing principal sensory nucleus of the trigeminal nerve: effect of transecting the infraorbital nerve. Brain Res. 1991 Sep 27;560(1-2):132–138. doi: 10.1016/0006-8993(91)91223-n. [DOI] [PubMed] [Google Scholar]
- Misra A. K., Menon N. K., Mishra S. K. Abnormal distribution of desmin and vimentin in myofibers in adult onset myotubular myopathy. Muscle Nerve. 1992 Nov;15(11):1246–1252. doi: 10.1002/mus.880151105. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Hoshi M., Nishida E., Minami Y., Sakai H. Binding of microtubule-associated protein 2 and tau to the intermediate filament reassembled from neurofilament 70-kDa subunit protein. Its regulation by calmodulin. J Biol Chem. 1986 Oct 5;261(28):13026–13030. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Erythrocyte microtubule assembly in vitro. Determination of the effects of erythrocyte tau, tubulin isoforms, and tubulin oligomers on erythrocyte tubulin assembly, and comparison with brain microtubule assembly. J Biol Chem. 1985 Oct 5;260(22):12293–12301. [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Padilla R., Maccioni R. B., Avila J. Calmodulin binds to a tubulin binding site of the microtubule-associated protein tau. Mol Cell Biochem. 1990 Sep 3;97(1):35–41. doi: 10.1007/BF00231699. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type. I. Morphology, evolution, distribution, and pathogenetic implications. Lab Invest. 1989 Jan;60(1):123–137. [PubMed] [Google Scholar]
- Pfeffer S. R., Drubin D. G., Kelly R. B. Identification of three coated vesicle components as alpha- and beta-tubulin linked to a phosphorylated 50,000-dalton polypeptide. J Cell Biol. 1983 Jul;97(1):40–47. doi: 10.1083/jcb.97.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer N. K., Walker R. A., Skeen V. P., Bourns B. D., Soboeiro M. F., Salmon E. D. Brain microtubule-associated proteins modulate microtubule dynamic instability in vitro. Real-time observations using video microscopy. J Cell Sci. 1992 Dec;103(Pt 4):965–976. doi: 10.1242/jcs.103.4.965. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., McKeel M., Hays T. Rapid rate of tubulin dissociation from microtubules in the mitotic spindle in vivo measured by blocking polymerization with colchicine. J Cell Biol. 1984 Sep;99(3):1066–1075. doi: 10.1083/jcb.99.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Six J., Lübke U., Mercken M., Vandermeeren M., Ceuterick C., Van de Voorde A., Boons J., Gheuens J. Specific monoclonal antibodies against normal microtubule-associated protein-2 (MAP2) epitopes present in Alzheimer pathological structures do not recognize paired helical filaments. Acta Neuropathol. 1992;83(2):179–189. doi: 10.1007/BF00308477. [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M., Jakes R., Klug A. Topographical relationship between beta-amyloid and tau protein epitopes in tangle-bearing cells in Alzheimer disease. Proc Natl Acad Sci U S A. 1990 May;87(10):3952–3956. doi: 10.1073/pnas.87.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell L. E., Edström L., Eriksson A., Henriksson K. G., Angqvist K. A. The distribution of intermediate filament protein (skeletin) in normal and diseased human skeletal muscle--an immunohistochemical and electron-microscopic study. J Neurol Sci. 1980 Aug;47(2):153–170. doi: 10.1016/0022-510x(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Toyama Y., Forry-Schaudies S., Hoffman B., Holtzer H. Effects of taxol and Colcemid on myofibrillogenesis. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6556–6560. doi: 10.1073/pnas.79.21.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of phosphate-independent MAP2 epitopes revealed with monoclonal antibodies in microwave-denatured human nervous system tissues. J Neurosci Methods. 1989 Aug;29(2):171–180. doi: 10.1016/0165-0270(89)90030-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989 Feb;37(2):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Valverde F., Lopez-Mascaraque L., De Carlos J. A. Distribution and morphology of Alz-50-immunoreactive cells in the developing visual cortex of kittens. J Neurocytol. 1990 Oct;19(5):662–671. doi: 10.1007/BF01188035. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Vandermeeren M., Mercken M., Vanmechelen E., Six J., van de Voorde A., Martin J. J., Cras P. Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993 Nov;61(5):1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Loomis P. A., Zinkowski R. P., Binder L. I. A novel tau transcript in cultured human neuroblastoma cells expressing nuclear tau. J Cell Biol. 1993 Apr;121(2):257–267. doi: 10.1083/jcb.121.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. H. Microtubular organization in elongating myogenic cells. J Cell Biol. 1974 Nov;63(2 Pt 1):550–566. doi: 10.1083/jcb.63.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Briones E., Koszka C., Artlieb U., Krepler R. Widespread occurrence of polypeptides related to neurotubule-associated proteins (MAP-1 and MAP-2) in non-neuronal cells and tissues. EMBO J. 1984 May;3(5):991–998. doi: 10.1002/j.1460-2075.1984.tb01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi P. S., Purich D. L. Microtubule-associated protein interactions with actin filaments: evidence for differential behavior of neuronal MAP-2 and tau in the presence of phosphatidyl-inositol. Biochem Biophys Res Commun. 1993 Feb 15;190(3):710–715. doi: 10.1006/bbrc.1993.1107. [DOI] [PubMed] [Google Scholar]