Abstract
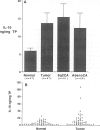
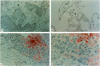
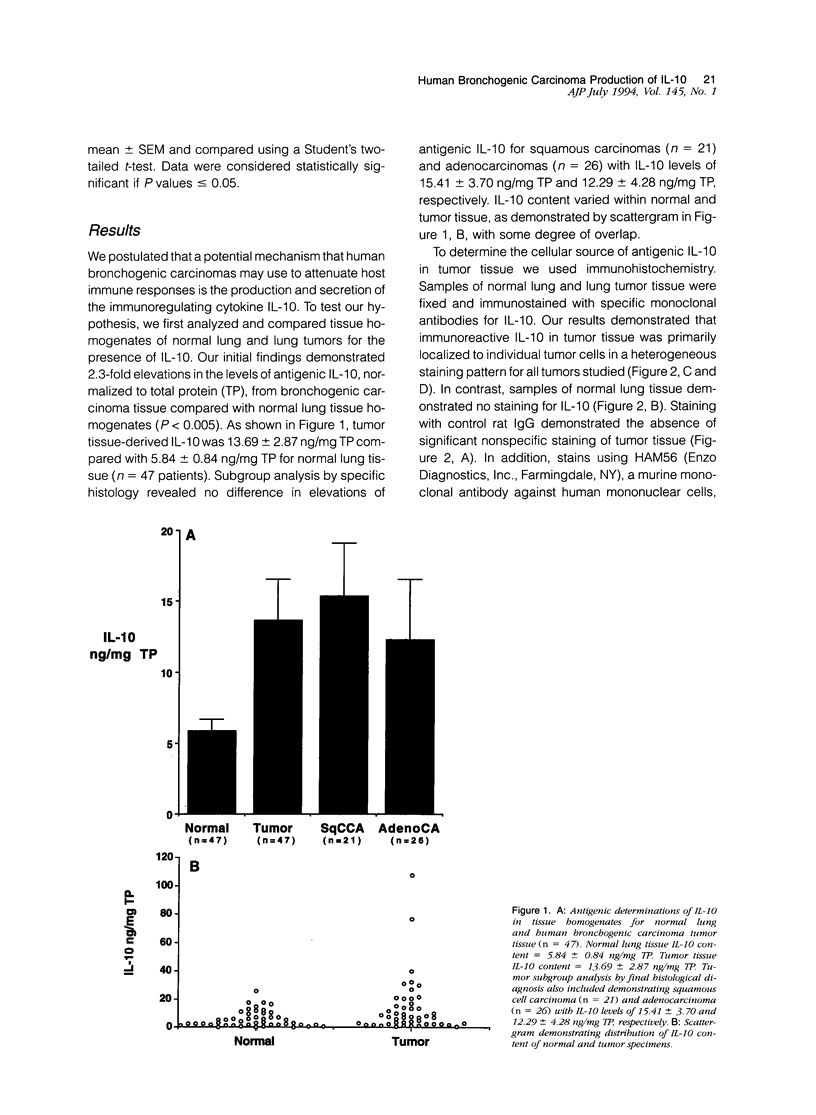
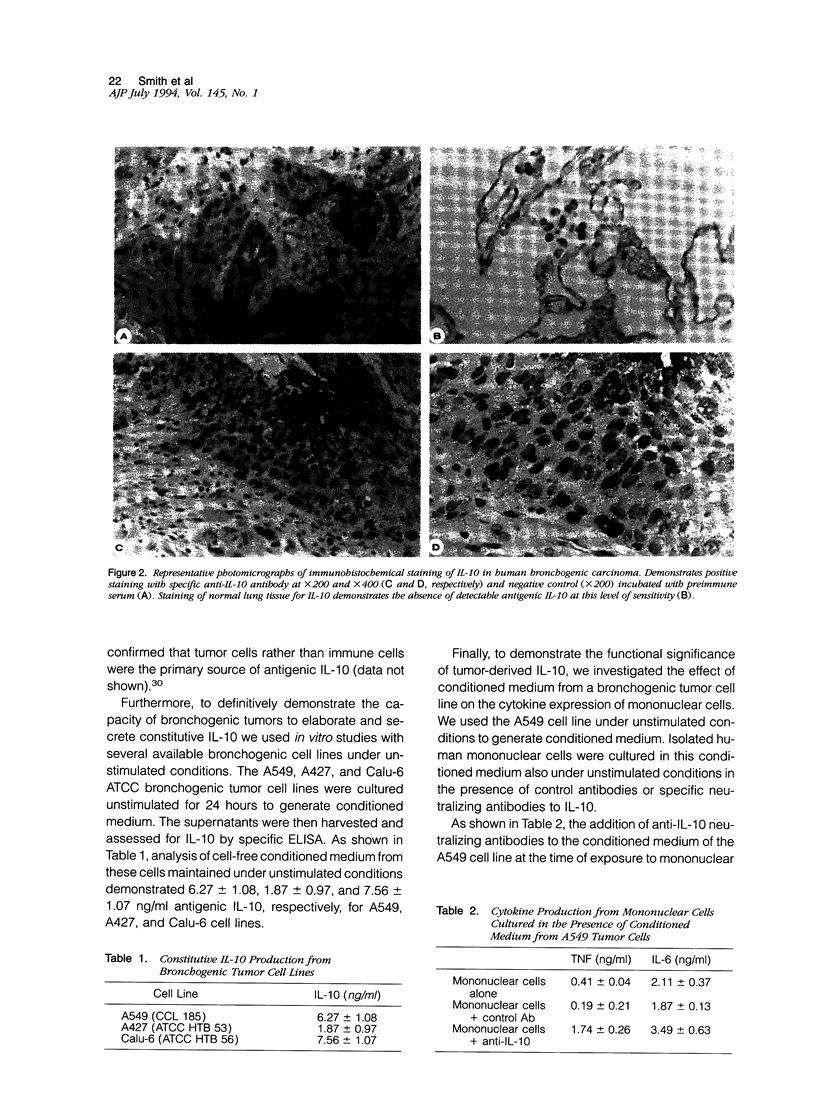
Interleukin-10 (IL-10) is a recently characterized cytokine with suppressive activity against various aspects of the cellular immune response. Our laboratory has previously demonstrated that another anti-inflammatory cytokine, IL-1 receptor antagonist (IRAP) is produced and secreted by human bronchogenic carcinomas. We speculated that tumor production of IRAP may mitigate host responses and confer increased tumor viability. In this study, we investigated the capacity of human bronchogenic tumors to produce IL-10 as another possible mechanism to attenuate host defenses. We found increased levels of antigenic IL-10 in tissue homogenates of human bronchogenic carcinomas compared with normal lung tissue (13.69 +/- 2.87 versus 5.84 +/- 0.84 ng/mg total protein). Immunohistochemical staining of tumors illustrate primary localization of antigenic IL-10 to individual tumor cells. Analysis of supernatants of several unstimulated human bronchogenic cell lines in vitro demonstrated the ability of tumor cells to constitutively produce IL-10. Functional studies of mononuclear cells, cultured in the presence of conditioned medium from a bronchogenic cell line, demonstrated their increased tumor necrosis factor and IL-6 production with the addition of neutralizing antibodies to IL-10. These findings demonstrate that human bronchogenic carcinomas elaborate functional IL-10, which may significantly impair immune effector cell function and enable the tumor to evade host defenses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon K. B., Westwick J., Camp R. D. Potent and specific inhibition of IL-8-, IL-1 alpha- and IL-1 beta-induced in vitro human lymphocyte migration by calcium channel antagonists. Biochem Biophys Res Commun. 1989 Nov 30;165(1):349–354. doi: 10.1016/0006-291x(89)91076-0. [DOI] [PubMed] [Google Scholar]
- Benjamin D., Knobloch T. J., Dayton M. A. Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt's lymphoma constitutively secrete large quantities of interleukin-10. Blood. 1992 Sep 1;80(5):1289–1298. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. F., Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991 Jul 15;147(2):528–534. [PubMed] [Google Scholar]
- Emilie D., Touitou R., Raphael M., Peuchmaur M., Devergnee O., Rea D., Coumbraras J., Crevon M. C., Edelman L., Joab I. In vivo production of interleukin-10 by malignant cells in AIDS lymphomas. Eur J Immunol. 1992 Nov;22(11):2937–2942. doi: 10.1002/eji.1830221127. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Burrows J. C., Evanoff H. L., Haines G. K., Pope R. M., Strieter R. M. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991 Oct 1;147(7):2187–2195. [PubMed] [Google Scholar]
- MacNeil I. A., Suda T., Moore K. W., Mosmann T. R., Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990 Dec 15;145(12):4167–4173. [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- O'Garra A., Chang R., Go N., Hastings R., Haughton G., Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992 Mar;22(3):711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Sampson-Johannes A., Fong S., Lowe D., Min H. Y., Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992 Feb 1;148(3):808–814. [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Kunkel S. L., Standiford T. J., Chensue S. W., Rolfe M. W., Orringer M. B., Whyte R. I., Burdick M. D., Danforth J. M., Gilbert A. R. The production of interleukin-1 receptor antagonist by human bronchogenic carcinoma. Am J Pathol. 1993 Sep;143(3):794–803. [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Thompson-Snipes L., Dhar V., Bond M. W., Mosmann T. R., Moore K. W., Rennick D. M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991 Feb 1;173(2):507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Modlin R. L., Ohmen J. D., Moy R. L. Local expression of antiinflammatory cytokines in cancer. J Clin Invest. 1993 Mar;91(3):1005–1010. doi: 10.1172/JCI116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]
- te Velde A. A., de Waal Malefijt R., Huijbens R. J., de Vries J. E., Figdor C. G. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992 Dec 15;149(12):4048–4052. [PubMed] [Google Scholar]