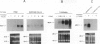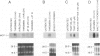Abstract
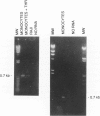
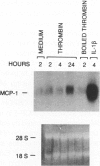
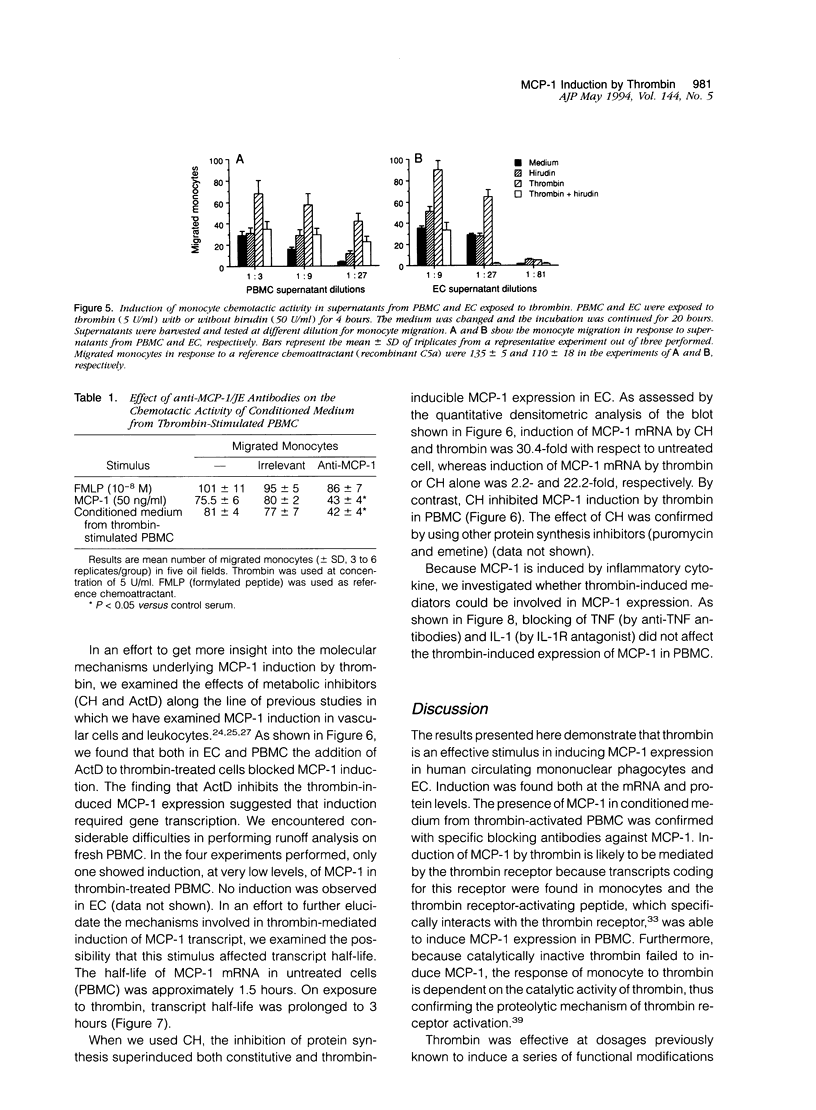
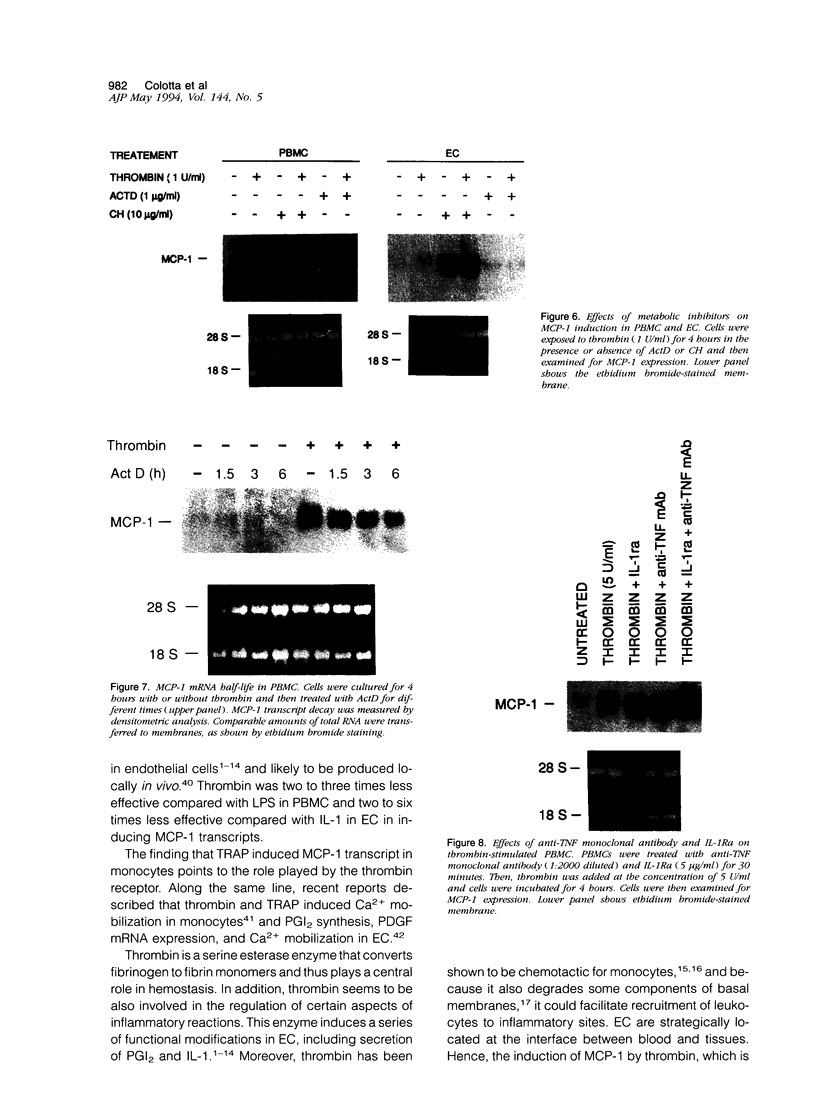
Thrombin, in addition to being a key enzyme in hemostasis, affects a series of endothelial and leukocyte functions and thus may be involved in the regulation of inflammatory reactions. Because leukocyte recruitment and activation are important events in inflammatory and thrombotic processes, in this study we have examined the possibility that thrombin induces the production of a cytokine chemotactic for mononuclear phagocytes. Human peripheral blood mononuclear cells (PBMC) exposed in vitro to thrombin expressed transcripts of monocyte chemotactic protein-1 (MCP-1; alternative acronyms: JE, monocyte chemotactic and activating factor, tumor-derived chemotactic factor). Thrombin was two- to threefold less effective than endotoxin in inducing MCP-1 transcripts in PBMC. Among circulating mononuclear cells, monocytes were identified as the cells expressing MCP-1 in response to thrombin. Monocytes expressed thrombin receptor transcripts. Boiling, hirudin, antithrombin III, and mutation of the catalytic site serine 205 into alanine) blocked the capacity of thrombin to induce MCP-1 expression. The thrombin receptor-activating peptide mimicked the effect of thrombin in inducing MCP-1 expression. Induction of MCP-1 transcript by thrombin was not reduced by blocking interleukin-1 and tumor necrosis factor, suggesting that these mediators are not involved in thrombin-induced expression of MCP-1. In addition to monocytes, endothelial cells (EC) also expressed MCP-1 in response to thrombin, although at lower levels compared with monocytes. Actinomycin D experiments indicated that induction of MCP-1 by thrombin in PBMC and EC was gene transcription dependent. The inhibition of protein synthesis blocked thrombin-induced MCP-1 expression in PBMC, whereas it superinduced both constitutive and thrombin-inducible expression of MCP-1 in EC, indicating different mechanisms of regulation of this gene in mononuclear phagocytes versus endothelial cells. Thrombin stimulated mononuclear cells and EC to release chemotactic activity for monocytes that could be inhibited by absorption with anti-MCP-1 antibodies. Induction of a chemotactic cytokine for monocytes by thrombin points to the importance of this enzyme in regulating inflammatory processes and further indicates that hemostasis, inflammation, and immunity are strictly interconnected processes.
Full text
PDF



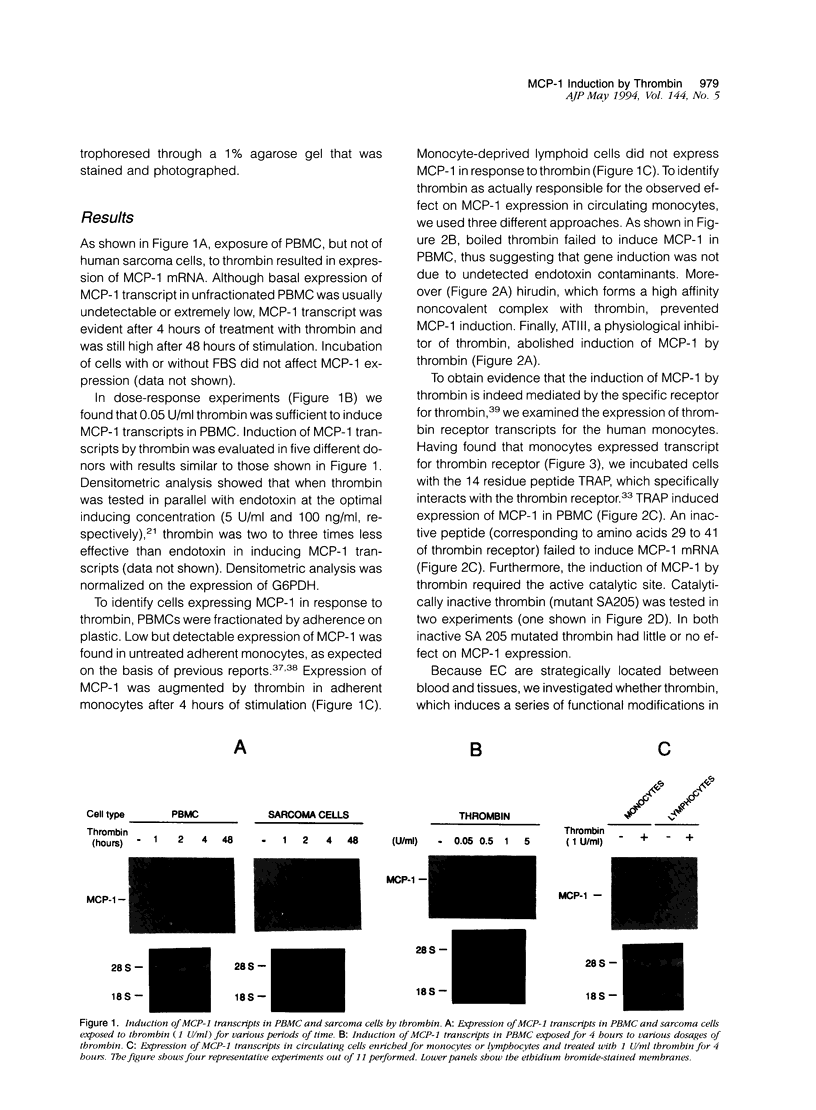
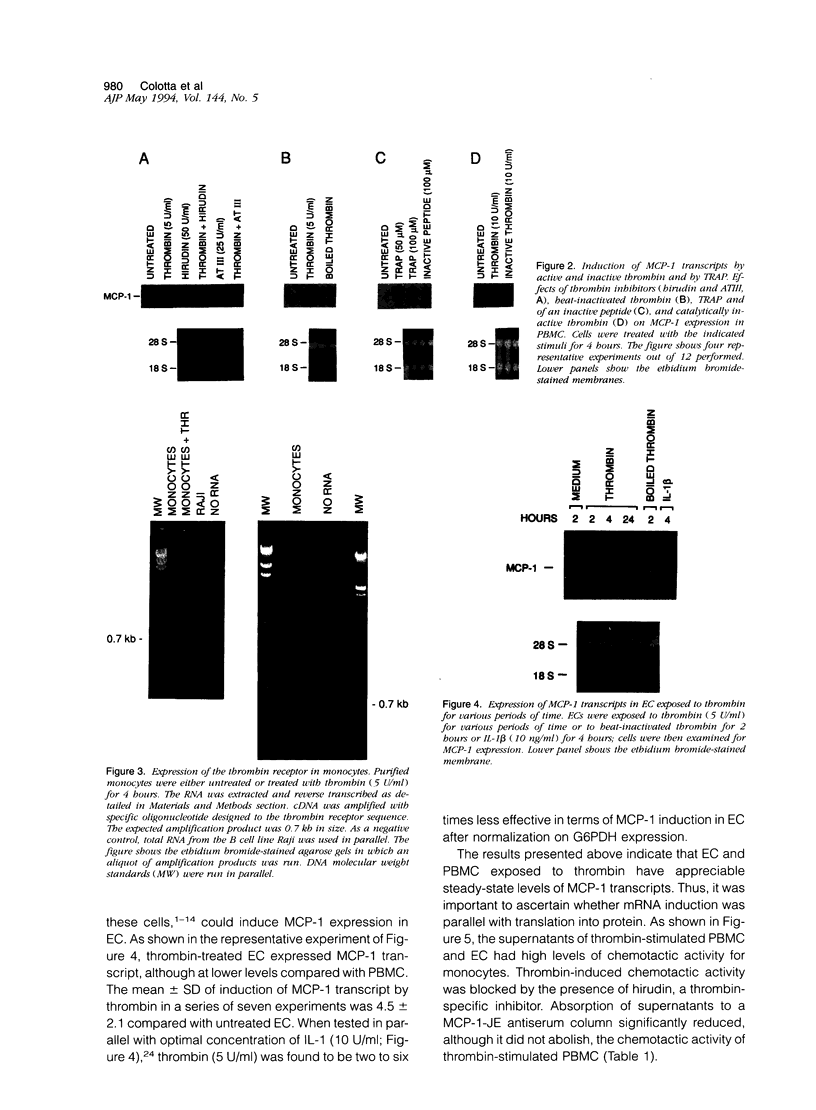



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983 Jan;96(1):282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colotta F., Borré A., Wang J. M., Tattanelli M., Maddalena F., Polentarutti N., Peri G., Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992 Feb 1;148(3):760–765. [PubMed] [Google Scholar]
- Coughlin S. R., Vu T. K., Hung D. T., Wheaton V. I. Characterization of a functional thrombin receptor. Issues and opportunities. J Clin Invest. 1992 Feb;89(2):351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin. Ann N Y Acad Sci. 1986;485:5–15. doi: 10.1111/j.1749-6632.1986.tb34563.x. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Nomura H., Notake M., Oyamada Y., Fukui T., Yamada M., Larsen C. G., Oppenheim J. J., Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem Biophys Res Commun. 1989 Feb 28;159(1):249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Painter R. G., Fenton J. W., 2nd, English D., Callahan K. S. Thrombin-induced prostacyclin biosynthesis in human endothelium: role of guanine nucleotide regulatory proteins in stimulus/coupling responses. J Cell Physiol. 1990 Jan;142(1):186–193. doi: 10.1002/jcp.1041420123. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Patterson C., Bahler C., Aschner J., Hart C. M., English D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis, and platelet-derived growth factor mRNA expression in cultured endothelium. J Cell Physiol. 1993 Sep;156(3):541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):165–169. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb R. H., Liotta L. A. Thrombin cleavage of extracellular matrix proteins. Ann N Y Acad Sci. 1986;485:288–292. doi: 10.1111/j.1749-6632.1986.tb34590.x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Brown K. D., Birdwell C. R., Zetter B. R. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978 Jun;77(3):774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988 Mar 1;140(5):1690–1694. [PubMed] [Google Scholar]
- Hoffman M., Church F. C. Response of blood leukocytes to thrombin receptor peptides. J Leukoc Biol. 1993 Aug;54(2):145–151. doi: 10.1002/jlb.54.2.145. [DOI] [PubMed] [Google Scholar]
- Huber P., Schmitz T., Griffin J., Jacobs M., Walsh C., Furie B., Furie B. C. Identification of amino acids in the gamma-carboxylation recognition site on the propeptide of prothrombin. J Biol Chem. 1990 Jul 25;265(21):12467–12473. [PubMed] [Google Scholar]
- Hébert C. A., Luscinskas F. W., Kiely J. M., Luis E. A., Darbonne W. C., Bennett G. L., Liu C. C., Obin M. S., Gimbrone M. A., Jr, Baker J. B. Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol. 1990 Nov 1;145(9):3033–3040. [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Colotta F., Polentarutti N., Pedenovi M., Mantovani A., Dejana E. Thrombin induces c-fos expression in cultured human endothelial cells by a Ca2(+)-dependent mechanism. Blood. 1990 Sep 15;76(6):1173–1180. [PubMed] [Google Scholar]
- Leonard E. J., Skeel A. Disposable microliter immunoabsorbent columns: construction and operation. J Immunol Methods. 1985 Oct 10;82(2):341–348. doi: 10.1016/0022-1759(85)90366-7. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Marzec U., Anderson J., Harker L. A. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 1984 Dec;74(6):1988–1995. doi: 10.1172/JCI111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Brand K., Edgington T. S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991 Dec 1;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J. JE/MCP-1: an early-response gene encodes a monocyte-specific cytokine. Cancer Cells. 1991 Dec;3(12):517–524. [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Sica A., Wang J. M., Colotta F., Dejana E., Mantovani A., Oppenheim J. J., Larsen C. G., Zachariae C. O., Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990 Apr 15;144(8):3034–3038. [PubMed] [Google Scholar]
- Sporn S. A., Eierman D. F., Johnson C. E., Morris J., Martin G., Ladner M., Haskill S. Monocyte adherence results in selective induction of novel genes sharing homology with mediators of inflammation and tissue repair. J Immunol. 1990 Jun 1;144(11):4434–4441. [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Sica A., Peri G., Walter S., Padura I. M., Libby P., Ceska M., Lindley I., Colotta F., Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 1991 Sep-Oct;11(5):1166–1174. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [PubMed] [Google Scholar]
- de Groot P. G., Gonsalves M. D., Loesberg C., van Buul-Wortelboer M. F., van Aken W. G., van Mourik J. A. Thrombin-induced release of von Willebrand factor from endothelial cells is mediated by phospholipid methylation. Prostacyclin synthesis is independent of phospholipid methylation. J Biol Chem. 1984 Nov 10;259(21):13329–13333. [PubMed] [Google Scholar]
- de Groot P. G., Reinders J. H., Sixma J. J. Perturbation of human endothelial cells by thrombin or PMA changes the reactivity of their extracellular matrix towards platelets. J Cell Biol. 1987 Mar;104(3):697–704. doi: 10.1083/jcb.104.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]