Abstract
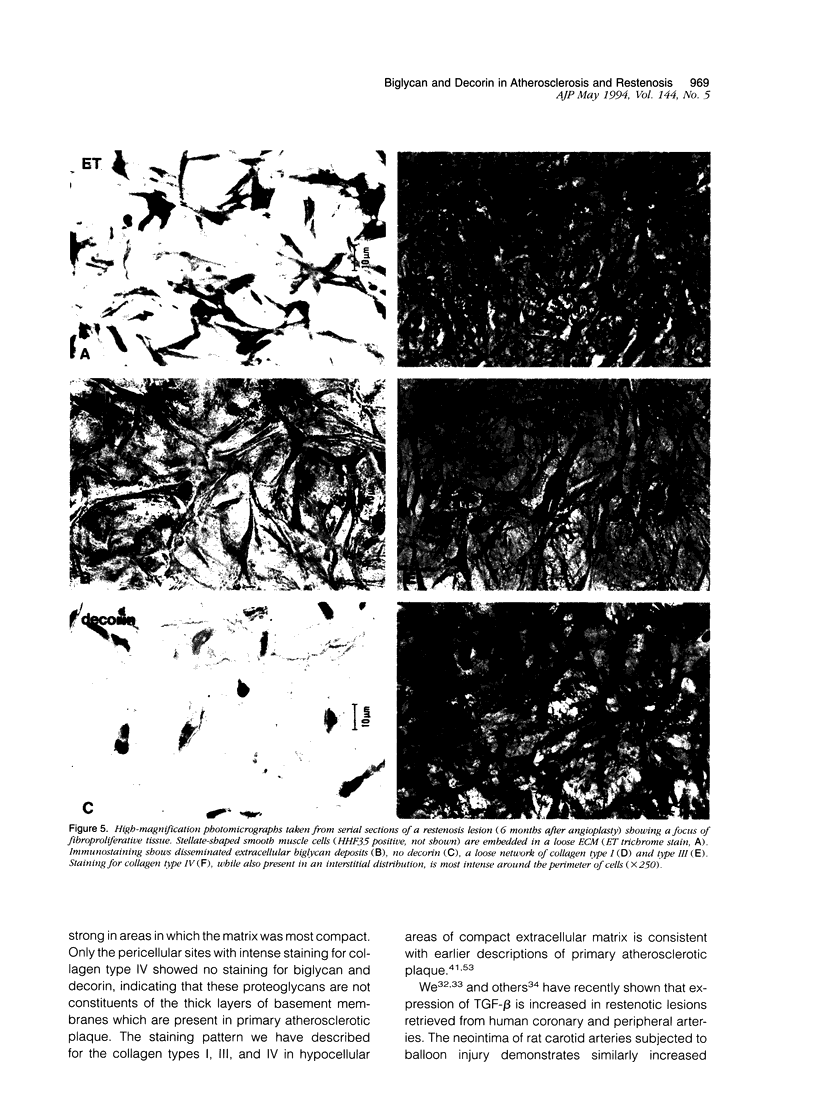
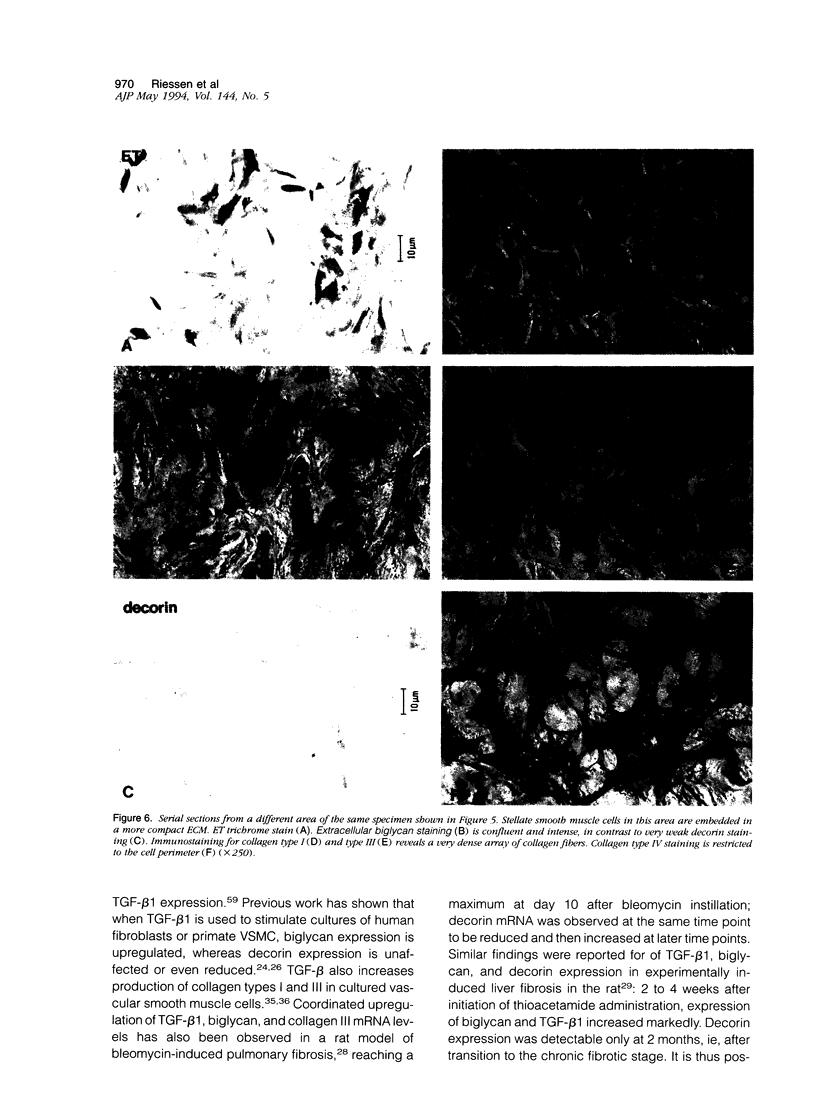
Proteoglycans are important constituents of blood vessels and accumulate in various forms of vascular disease. Little is known concerning the proteoglycan composition of restenotic lesions formed after angioplasty and whether the proteoglycan composition of these lesions differs from that of primary atherosclerosis. Accordingly, we sought to characterize the distribution of two proteoglycans, biglycan and decorin, in primary atherosclerotic and restenotic lesions of human coronary arteries. Restenosis (n = 37) and primary (n = 11) lesions obtained from 48 patients by directional atherectomy of human coronary arteries were stained with antibodies against biglycan and decorin. To further characterize the extracellular matrix of restenotic tissues, we studied the co-distribution of these proteoglycans with collagen types I, III, and IV. The loose fibroproliferative tissue seen predominantly in restenosis lesions consistently stained positively for biglycan in patterns of deposition ranging from disseminated to homogeneous. The density and intensity of biglycan staining was correlated with the density of collagen type I and III fiber networks, both of which were observed to interweave among the loose fibroproliferative tissue. The compact connective tissue of primary atherosclerotic plaque was characterized by strong biglycan staining which co-localized with intense collagen type I and III staining. Only basement membrane-like structures rich in collagen type IV demonstrated negative biglycan staining. In contrast, loose fibroproliferative tissue exhibited no significant staining for decorin. Strong immunostaining for decorin, however, was found in primary atherosclerotic plaque. There are thus regional differences in the distribution of extracellular matrix proteoglycans of restenotic and primary human atherosclerotic lesions; these observations suggest that differences established for the biological roles of biglycan and decorin in other organ systems may extend as well to pathologically altered human coronary arteries.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Regulation of development and differentiation by the extracellular matrix. Development. 1993 Apr;117(4):1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Amento E. P., Ehsani N., Palmer H., Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991 Sep-Oct;11(5):1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. D., Kniep A. C., Pierce R. A., Deak S. B., Karboski C., Miller D. C., Parker M. I., Mackenzie J. W., Rosenbloom J., Scott G. E. Increased elastin mRNA levels associated with surgically induced intimal injury. Connect Tissue Res. 1988;18(2):65–78. doi: 10.3109/03008208809008059. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Vogel K. G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9(6):468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Edwards I. J., Wagner W. D., Owens R. T. Macrophage secretory products selectively stimulate dermatan sulfate proteoglycan production in cultured arterial smooth muscle cells. Am J Pathol. 1990 Mar;136(3):609–621. [PMC free article] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Garratt K. N., Edwards W. D., Kaufmann U. P., Vlietstra R. E., Holmes D. R., Jr Differential histopathology of primary atherosclerotic and restenotic lesions in coronary arteries and saphenous vein bypass grafts: analysis of tissue obtained from 73 patients by directional atherectomy. J Am Coll Cardiol. 1991 Feb;17(2):442–448. doi: 10.1016/s0735-1097(10)80113-5. [DOI] [PubMed] [Google Scholar]
- Godfrey M., Keene D. R., Blank E., Hori H., Sakai L. Y., Sherwin L. A., Hollister D. W. Type II achondrogenesis-hypochondrogenesis: morphologic and immunohistopathologic studies. Am J Hum Genet. 1988 Dec;43(6):894–903. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Heino J., Kähäri V. M., Mauviel A., Krusius T. Human recombinant interleukin-1 regulates cellular mRNA levels of dermatan sulphate proteoglycan core protein. Biochem J. 1988 May 15;252(1):309–312. doi: 10.1042/bj2520309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järveläinen H. T., Kinsella M. G., Wight T. N., Sandell L. J. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991 Dec 5;266(34):23274–23281. [PubMed] [Google Scholar]
- Katsuda S., Okada Y., Minamoto T., Oda Y., Matsui Y., Nakanishi I. Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler Thromb. 1992 Apr;12(4):494–502. doi: 10.1161/01.atv.12.4.494. [DOI] [PubMed] [Google Scholar]
- Krull N. B., Zimmermann T., Gressner A. M. Spatial and temporal patterns of gene expression for the proteoglycans biglycan and decorin and for transforming growth factor-beta 1 revealed by in situ hybridization during experimentally induced liver fibrosis in the rat. Hepatology. 1993 Sep;18(3):581–589. [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Liau G., Chan L. M. Regulation of extracellular matrix RNA levels in cultured smooth muscle cells. Relationship to cellular quiescence. J Biol Chem. 1989 Jun 15;264(17):10315–10320. [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh K. G., Duance V. C., Bishop K. A. The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol. 1980 Jan;130(1):45–55. doi: 10.1002/path.1711300107. [DOI] [PubMed] [Google Scholar]
- McCullagh K. G. Increased type I collagen in human atherosclerotic plaque. Atherosclerosis. 1983 Feb;46(2):247–248. doi: 10.1016/0021-9150(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Scott L. J. Effects of endothelial removal and regeneration on smooth muscle glycosaminoglycan synthesis and growth in rat carotid artery in organ culture. Lab Invest. 1985 Apr;52(4):409–419. [PubMed] [Google Scholar]
- Miller M. J., Kuntz R. E., Friedrich S. P., Leidig G. A., Fishman R. F., Schnitt S. J., Baim D. S., Safian R. D. Frequency and consequences of intimal hyperplasia in specimens retrieved by directional atherectomy of native primary coronary artery stenoses and subsequent restenoses. Am J Cardiol. 1993 Mar 15;71(8):652–658. doi: 10.1016/0002-9149(93)91005-3. [DOI] [PubMed] [Google Scholar]
- Morton L. F., Barnes M. J. Collagen polymorphism in the normal and diseased blood vessel wall. Investigation of collagens types I, III and V. Atherosclerosis. 1982 Mar;42(1):41–51. doi: 10.1016/0021-9150(82)90124-1. [DOI] [PubMed] [Google Scholar]
- Murata K., Motayama T., Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis. 1986 Jun;60(3):251–262. doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol S., Isner J. M., Pickering J. G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992 Oct;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuyoshi M., Kimura T., Ohishi H., Horiuchi H., Nosaka H., Hamasaki N., Yokoi H., Kim K. Restenosis after percutaneous transluminal coronary angioplasty: pathologic observations in 20 patients. J Am Coll Cardiol. 1991 Feb;17(2):433–439. doi: 10.1016/s0735-1097(10)80111-1. [DOI] [PubMed] [Google Scholar]
- Odermatt B. F., Lang A. B., Rüttner J. R., Winterhalter K. H., Trüeb B. Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7343–7347. doi: 10.1073/pnas.81.23.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Ihnatowycz I., Moore S. Glycosaminoglycan distribution in rabbit aortic wall following balloon catheter deendothelialization. An ultrastructural study. Lab Invest. 1980 Dec;43(6):509–516. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Safian R. D., Gelbfish J. S., Erny R. E., Schnitt S. J., Schmidt D. A., Baim D. S. Coronary atherectomy. Clinical, angiographic, and histological findings and observations regarding potential mechanisms. Circulation. 1990 Jul;82(1):69–79. doi: 10.1161/01.cir.82.1.69. [DOI] [PubMed] [Google Scholar]
- Salisbury B. G., Hajjar D. P., Minick C. R. Altered glycosaminoglycan metabolism in injured arterial wall. Exp Mol Pathol. 1985 Jun;42(3):306–319. doi: 10.1016/0014-4800(85)90081-4. [DOI] [PubMed] [Google Scholar]
- Schlumberger W., Thie M., Rauterberg J., Robenek H. Collagen synthesis in cultured aortic smooth muscle cells. Modulation by collagen lattice culture, transforming growth factor-beta 1, and epidermal growth factor. Arterioscler Thromb. 1991 Nov-Dec;11(6):1660–1666. doi: 10.1161/01.atv.11.6.1660. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Schönherr E., Järveläinen H. T., Kinsella M. G., Sandell L. J., Wight T. N. Platelet-derived growth factor and transforming growth factor-beta 1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler Thromb. 1993 Jul;13(7):1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Muzykantov V. R., Idelson G. L., Rukosuev V. S. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. 1985 Sep;5(4):355–368. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- Simpson J. B., Selmon M. R., Robertson G. C., Cipriano P. R., Hayden W. G., Johnson D. E., Fogarty T. J. Transluminal atherectomy for occlusive peripheral vascular disease. Am J Cardiol. 1988 May 9;61(14):96G–101G. doi: 10.1016/s0002-9149(88)80040-7. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Nakashima N., Fukatsu T., Nagasaka T., Katoh T., Ogura T., Takeuchi J. Production and characterization of monoclonal antibody to dermatan sulfate proteoglycan. J Histochem Cytochem. 1988 May;36(5):479–485. doi: 10.1177/36.5.3356894. [DOI] [PubMed] [Google Scholar]
- Stary H. C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990 Aug;11 (Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Schmidhauser C., Kobrin M., Bissell M. J., Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993 Jan;120(1):253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöcker G., Meyer H. E., Wagener C., Greiling H. Purification and N-terminal amino acid sequence of a chondroitin sulphate/dermatan sulphate proteoglycan isolated from intima/media preparations of human aorta. Biochem J. 1991 Mar 1;274(Pt 2):415–420. doi: 10.1042/bj2740415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Tsukada T., Numano F., Fujimoto T. Fibrocellular tissue response after percutaneous transluminal coronary angioplasty. An immunocytochemical analysis of the cellular composition. Circulation. 1991 Apr;83(4):1327–1332. doi: 10.1161/01.cir.83.4.1327. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Hernandez D. J. The effects of transforming growth factor-beta and serum on proteoglycan synthesis by tendon fibrocartilage. Eur J Cell Biol. 1992 Dec;59(2):304–313. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Hernnäs J., Särnstrand B., Oldberg A., Heinegård D., Malmström A. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest. 1993 Aug;92(2):632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Curwen K. D., Litrenta M. M., Alonso D. R., Minick C. R. Effect of endothelium on glycosaminoglycan accumulation in injured rabbit aorta. Am J Pathol. 1983 Nov;113(2):156–164. [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Kinsella M. G., Qwarnström E. E. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol. 1992 Oct;4(5):793–801. doi: 10.1016/0955-0674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Yeo T. K., Brown L., Dvorak H. F. Alterations in proteoglycan synthesis common to healing wounds and tumors. Am J Pathol. 1991 Jun;138(6):1437–1450. [PMC free article] [PubMed] [Google Scholar]