Abstract
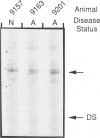
The Korat cat provides an animal model for type II GM2-gangliosidosis (Sandhoff disease) that may be suitable for tests of gene replacement therapy with the HEXB gene encoding the beta subunit of the beta-hexosaminidases. In the present report, we examined the brain and liver pathology of a typical Sandhoff-affected cat. We characterized the feline HEXB complementary DNA (cDNA) and determined the molecular defect in this feline model. cDNA libraries were produced from one normal and one affected animal, and cDNA clones homologous to human HEXB were sequenced. In the affected cDNA clone, the deletion of a cytosine residue at position +39 of the putative coding region results in a frame shift and a stop codon at base +191. This disease-related deletion was consistently detected by sequencing of cloned polymerase chain reaction amplified reverse transcribed messenger RNA from one more normal Korat and two additional affected animals. The defect was further demonstrated using single-strand conformational polymorphism analysis of the polymerase chain reaction products. In addition, alternative splicing of both normal and affected messenger RNAs was demonstrated. These results should facilitate the use of this animal model to assess gene therapy.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Chelly J., Mezard C., Gandy S., Kahn A., Poenaru L. A "G" to "A" mutation at position -1 of a 5' splice site in a late infantile form of Tay-Sachs disease. J Biol Chem. 1990 May 5;265(13):7324–7330. [PubMed] [Google Scholar]
- Baker H. J., Mole J. A., Lindsey J. R., Creel R. M. Animal models of human ganglioside storage diseases. Fed Proc. 1976 Apr;35(5):1193–1201. [PubMed] [Google Scholar]
- Chiocca E. A., Choi B. B., Cai W. Z., DeLuca N. A., Schaffer P. A., DiFiglia M., Breakefield X. O., Martuza R. L. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 1990 Aug;2(8):739–746. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Munnell J. F., Lorenz M. D., Murphy J. V., Baker H. J., Rattazzi M. C. GM2 ganglioside lysosomal storage disease in cats with beta-hexosaminidase deficiency. Science. 1977 May 27;196(4293):1014–1017. doi: 10.1126/science.404709. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Lau M. M., Neufeld E. F. A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the alpha-subunit of beta-hexosaminidase. J Biol Chem. 1989 Dec 15;264(35):21376–21380. [PubMed] [Google Scholar]
- Mahuran D. J. The biochemistry of HEXA and HEXB gene mutations causing GM2 gangliosidosis. Biochim Biophys Acta. 1991 Feb 22;1096(2):87–94. doi: 10.1016/0925-4439(91)90044-a. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Costigan F. C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J Biol Chem. 1988 Dec 15;263(35):18587–18589. [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Suzuki K. Genetic cause of a juvenile form of Sandhoff disease. Abnormal splicing of beta-hexosaminidase beta chain gene transcript due to a point mutation within intron 12. J Biol Chem. 1989 Mar 25;264(9):5155–5158. [PubMed] [Google Scholar]
- Neote K., Bapat B., Dumbrille-Ross A., Troxel C., Schuster S. M., Mahuran D. J., Gravel R. A. Characterization of the human HEXB gene encoding lysosomal beta-hexosaminidase. Genomics. 1988 Nov;3(4):279–286. doi: 10.1016/0888-7543(88)90116-4. [DOI] [PubMed] [Google Scholar]
- Neote K., Brown C. A., Mahuran D. J., Gravel R. A. Translation initiation in the HEXB gene encoding the beta-subunit of human beta-hexosaminidase. J Biol Chem. 1990 Dec 5;265(34):20799–20806. [PubMed] [Google Scholar]
- Neufeld E. F. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Pagel M. A., Dix R. D. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood-brain barrier. J Neurosurg. 1991 Mar;74(3):475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Palella T. D., Hidaka Y., Silverman L. J., Levine M., Glorioso J., Kelley W. N. Expression of human HPRT mRNA in brains of mice infected with a recombinant herpes simplex virus-1 vector. Gene. 1989 Aug 1;80(1):137–144. doi: 10.1016/0378-1119(89)90258-8. [DOI] [PubMed] [Google Scholar]
- Proia R. L. Gene encoding the human beta-hexosaminidase beta chain: extensive homology of intron placement in the alpha- and beta-chain genes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1883–1887. doi: 10.1073/pnas.85.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork P., Loda M., Bosari S., Wiley B., Poppenhusen K., Wolfe H. Detection of K-ras mutations in pancreatic and hepatic neoplasms by non-isotopic mismatched polymerase chain reaction. Oncogene. 1991 May;6(5):857–862. [PubMed] [Google Scholar]
- Triggs-Raine B. L., Akerman B. R., Clarke J. T., Gravel R. A. Sequence of DNA flanking the exons of the HEXA gene, and identification of mutations in Tay-Sachs disease. Am J Hum Genet. 1991 Nov;49(5):1041–1054. [PMC free article] [PubMed] [Google Scholar]
- Walkley S. U., Baker H. J., Rattazzi M. C. Initiation and growth of ectopic neurites and meganeurites during postnatal cortical development in ganglioside storage disease. Brain Res Dev Brain Res. 1990 Feb 1;51(2):167–178. doi: 10.1016/0165-3806(90)90273-2. [DOI] [PubMed] [Google Scholar]