Abstract
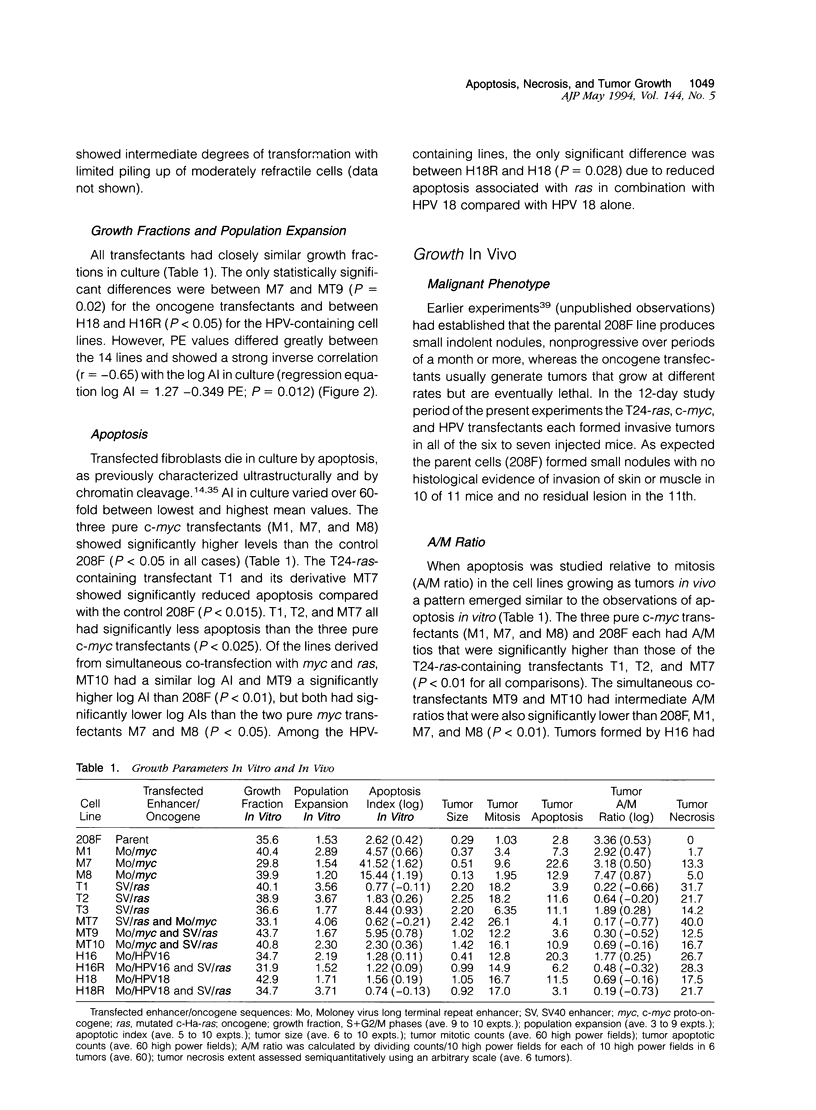
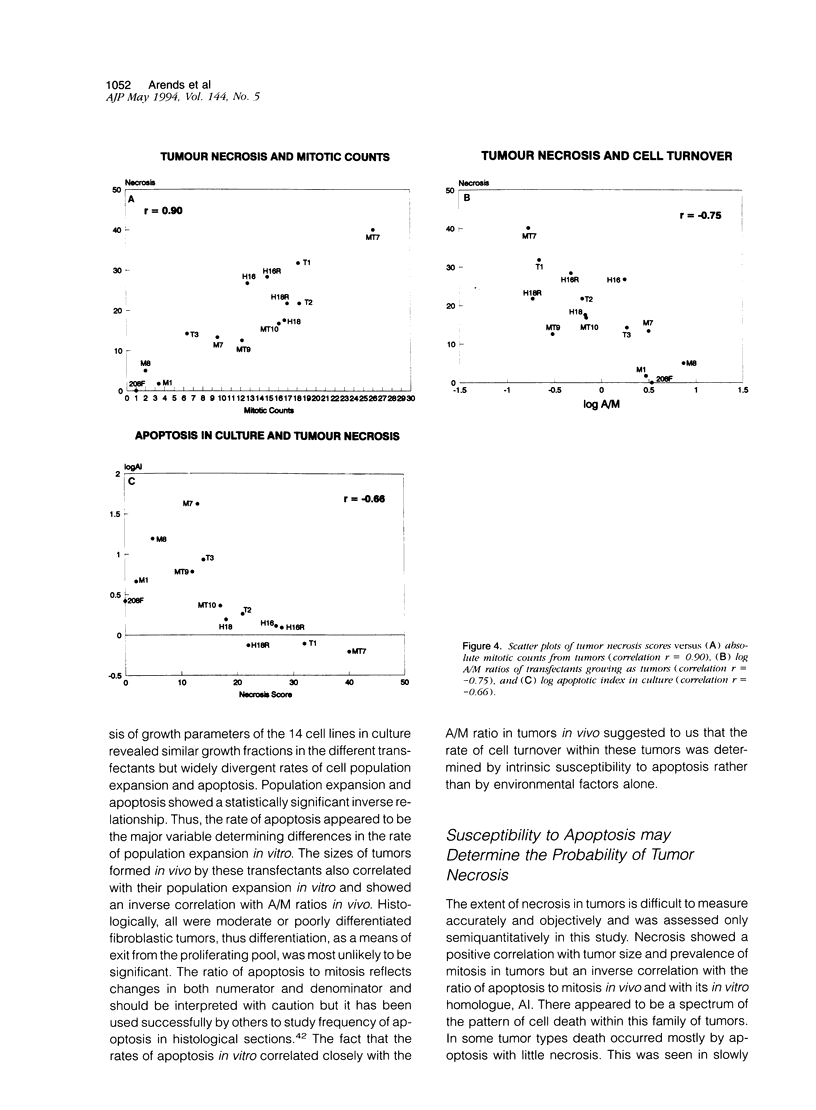
Immortalized rat fibroblasts were transfected with expression plasmids containing a mutated human Ha-ras (T24) oncogene, human c-myc, HPV 16 or 18 genomes, or combinations of these. Cell proliferation rates in vitro of the resulting 13 transformed lines were closely similar but apoptotic rates in vitro varied over a 60-fold range and correlated inversely with rates of population expansion in culture. To determine whether such differences in susceptibility to apoptosis affected the pattern of tumor growth in vivo, the transfected lines were injected subcutaneously into immunesuppressed mice producing fibrosarcomas in which prevalence of apoptosis and mitosis, extent of necrosis, and net growth rate were measured. Cell lines with high apoptotic rates in vitro tended to generate slowly growing tumors with high ratios of apoptosis to mitosis and little necrosis. The three most extreme examples of this phenotype all resulted from single transfections with c-myc. Lines with low apoptotic rates in vitro generated rapidly expanding tumors with high mitotic rates, extensive necrosis, and little apoptosis relative to mitosis, even in the compromised zone at the edge of necrotic regions. The four fastest-growing tumors all contained a T24-ras oncogene. The results suggest that oncogene expression determines intrinsic apoptotic rates and in this way may significantly influence the net growth rate and extent of necrosis in tumors.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D. J., Howell A., Roberts S. A., Williams G. T., Watson R. J., Coyne J. D., Clarke R. B., Laidlaw I. J., Potten C. S. Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human breast. J Pathol. 1992 May;167(1):25–32. doi: 10.1002/path.1711670106. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Donaldson Y. K., Duvall E., Wyllie A. H., Bird C. C. HPV in full thickness cervical biopsies: high prevalence in CIN 2 and CIN 3 detected by a sensitive PCR method. J Pathol. 1991 Dec;165(4):301–309. doi: 10.1002/path.1711650405. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Donaldson Y. K., Duvall E., Wyllie A. H., Bird C. C. Human papillomavirus type 18 associates with more advanced cervical neoplasia than human papillomavirus type 16. Hum Pathol. 1993 Apr;24(4):432–437. doi: 10.1016/0046-8177(93)90093-v. [DOI] [PubMed] [Google Scholar]
- Arends M. J., McGregor A. H., Toft N. J., Brown E. J., Wyllie A. H. Susceptibility to apoptosis is differentially regulated by c-myc and mutated Ha-ras oncogenes and is associated with endonuclease availability. Br J Cancer. 1993 Dec;68(6):1127–1133. doi: 10.1038/bjc.1993.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H., Bird C. C. Papillomaviruses and human cancer. Hum Pathol. 1990 Jul;21(7):686–698. doi: 10.1016/0046-8177(90)90027-3. [DOI] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Baak J. P. Mitosis counting in tumors. Hum Pathol. 1990 Jul;21(7):683–685. doi: 10.1016/0046-8177(90)90026-2. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Sarang M., Jenkin J., Kerrigan D., Pommier Y. Differential induction of secondary DNA fragmentation by topoisomerase II inhibitors in human tumor cell lines with amplified c-myc expression. Cancer Res. 1991 Dec 1;51(23 Pt 1):6280–6285. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Evans C. A., Owen-Lynch P. J., Whetton A. D., Dive C. Activation of the Abelson tyrosine kinase activity is associated with suppression of apoptosis in hemopoietic cells. Cancer Res. 1993 Apr 15;53(8):1735–1738. [PubMed] [Google Scholar]
- Evans D. L., Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993 May 1;53(9):2133–2139. [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Franko A. J., Sutherland R. M. Oxygen diffusion distance and development of necrosis in multicell spheroids. Radiat Res. 1979 Sep;79(3):439–453. [PubMed] [Google Scholar]
- Franko A. J., Sutherland R. M. Radiation survival of cells from spheroids grown in different oxygen concentrations. Radiat Res. 1979 Sep;79(3):454–467. [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Joiner B., Hirst V. K. Blood flow modification by nicotinamide and metoclopramide in mouse tumours growing in different sites. Br J Cancer. 1993 Jan;67(1):1–6. doi: 10.1038/bjc.1993.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jones B., Camplejohn R. S. Stathmokinetic measurement of tumour cell proliferation in relation to vascular proximity. Cell Tissue Kinet. 1983 Jul;16(4):351–355. [PubMed] [Google Scholar]
- Kerr J. F., Searle J. A suggested explanation for the paradoxically slow growth rate of basal-cell carcinomas that contain numerous mitotic figures. J Pathol. 1972 May;107(1):41–44. doi: 10.1002/path.1711070107. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K. M., Lamb D. Actual growth rate and tumour cell proliferation in human pulmonary neoplasms. Br J Cancer. 1984 Sep;50(3):343–349. doi: 10.1038/bjc.1984.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Münger K., Yee C. L., Phelps W. C., Pietenpol J. A., Moses H. L., Howley P. M. Biochemical and biological differences between E7 oncoproteins of the high- and low-risk human papillomavirus types are determined by amino-terminal sequences. J Virol. 1991 Jul;65(7):3943–3948. doi: 10.1128/jvi.65.7.3943-3948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Quinn C. M., Wright N. A. The clinical assessment of proliferation and growth in human tumours: evaluation of methods and applications as prognostic variables. J Pathol. 1990 Feb;160(2):93–102. doi: 10.1002/path.1711600202. [DOI] [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Sarraf C. E., Bowen I. D. Kinetic studies on a murine sarcoma and an analysis of apoptosis. Br J Cancer. 1986 Dec;54(6):989–998. doi: 10.1038/bjc.1986.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf C. E., Bowen I. D. Proportions of mitotic and apoptotic cells in a range of untreated experimental tumours. Cell Tissue Kinet. 1988 Jan;21(1):45–49. doi: 10.1111/j.1365-2184.1988.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shan X., Aw T. Y., Smith E. R., Ingelman-Sundberg M., Mannervik B., Iyanagi T., Jones D. P. Effect of chronic hypoxia on detoxication enzymes in rat liver. Biochem Pharmacol. 1992 Jun 9;43(11):2421–2426. doi: 10.1016/0006-2952(92)90322-a. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer G. M., Lewis S., Michalowski A., Aber V. The relationship between regional variations in blood flow and histology in a transplanted rat fibrosarcoma. Br J Cancer. 1990 Feb;61(2):250–257. doi: 10.1038/bjc.1990.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S. Cytotoxic T lymphocytes and glucocorticoids activate an endogenous suicide process in target cells. Nature. 1987 May 7;327(6117):62–64. doi: 10.1038/327062a0. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. Standardization of high-resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry. 1983 Mar;3(5):328–331. doi: 10.1002/cyto.990030504. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis: cell death under homeostatic control. Arch Toxicol Suppl. 1987;11:3–10. doi: 10.1007/978-3-642-72558-6_1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Arends M. J., Morris R. G., Walker S. W., Evan G. The apoptosis endonuclease and its regulation. Semin Immunol. 1992 Dec;4(6):389–397. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Rose K. A., Morris R. G., Steel C. M., Foster E., Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: growth, metastasis and endogenous oncogene expression. Br J Cancer. 1987 Sep;56(3):251–259. doi: 10.1038/bjc.1987.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. The biology of cell death in tumours. Anticancer Res. 1985 Jan-Feb;5(1):131–136. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]




