Abstract
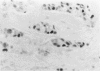
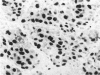
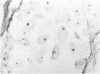
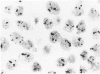
The proliferative activity of male breast carcinoma has been investigated using the staining of the argyrophilic nucleolar organizer regions (AgNORs), the monoclonal antibody against the proliferating cell nuclear antigen (PC10) and the monoclonal antibody MIB-1 in formalin-fixed, paraffin-embedded specimens from 27 primary male breast carcinomas at diagnosis. A significant correlation was found between survival and AgNOR counts (median of survival 77 months for cases with AgNOR/cell < or = 7.27 but 37 months only for cases with > 7.27 AgNOR/cell; P = 0.001), proliferating cell nuclear antigen scores (median of survival 73 months for cases with proliferating cell nuclear antigen < or = 18.25% versus 41 for cases with proliferating cell nuclear antigen > 18.25%; P = 0.013) and MIB-1 scores (median of survival 73 months for cases with MIB-1 scores < or = 23.5% versus 37 months for cases with MIB-1 scores > 23.5%; P = 0.01). Tumor histological grade was also correlated with prognosis (median of survival 72 months for grade 2 versus 33 months for grade 3 tumors; P = 0.01). Estrogen and progesterone receptors, immunohistochemically detected on paraffin-embedded sections, had no prognostic value. In the multivariate survival analysis, only AgNOR counts (P = 0.007) and tumor size (P = 0.003) had an independent prognostic significance. Our results indicate that methods for assessing the cell proliferation in routinely processed specimens offer significant prognostic information in male breast carcinoma. The finding, together with the lack of prognostic significance for estrogen receptors and progesterone receptors, suggests that male breast carcinoma is biologically different from female breast cancer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltomaa S., Lipponen P., Papinaho S., Syrjänen K. Proliferating-cell nuclear antigen (PC10) immunolabelling and other proliferation indices as prognostic factors in breast cancer. J Cancer Res Clin Oncol. 1993;119(5):288–294. doi: 10.1007/BF01212727. [DOI] [PubMed] [Google Scholar]
- Aaltomaa S., Lipponen P., Syrjänen K. Nucleolar organizer regions related to morphometry, flow cytometry, sex steroid receptor content, tumour histology and prognosis in female breast cancer. Pathol Res Pract. 1993 May;189(4):416–421. doi: 10.1016/S0344-0338(11)80329-8. [DOI] [PubMed] [Google Scholar]
- Alanko A., Heinonen E., Scheinin T., Tolppanen E. M., Vihko R. Significance of estrogen and progesterone receptors, disease-free interval, and site of first metastasis on survival of breast cancer patients. Cancer. 1985 Oct 1;56(7):1696–1700. doi: 10.1002/1097-0142(19851001)56:7<1696::aid-cncr2820560738>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Andersen J., Thorpe S. M., King W. J., Rose C., Christensen I., Rasmussen B. B., Poulsen H. S. The prognostic value of immunohistochemical estrogen receptor analysis in paraffin-embedded and frozen sections versus that of steroid-binding assays. Eur J Cancer. 1990 Apr;26(4):442–449. doi: 10.1016/0277-5379(90)90013-j. [DOI] [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzubar N., Walker K. J., Griffiths K., Ellis I. O., Elston C. W., Robertson J. F., Blamey R. W., Nicholson R. I. Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer. 1989 Jun;59(6):943–947. doi: 10.1038/bjc.1989.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Charpin C., Bonnier P., Piana L., Kouzhami H., Devictor B., Lavaut M. N., Andrac L., Allasia C. Correlation of nucleolar organizer regions and nuclear morphometry assessed by automatic image analysis in breast cancer with aneuploidy, K167 immunostaining, histopathologic grade and lymph node involvement. Pathol Res Pract. 1992 Dec;188(8):1009–1017. doi: 10.1016/s0344-0338(11)81245-8. [DOI] [PubMed] [Google Scholar]
- Ciatto S., Iossa A., Bonardi R., Pacini P. Male breast carcinoma: review of a multicenter series of 150 cases. Coordinating Center and Writing Committee of FONCAM (National Task Force for Breast Cancer), Italy. Tumori. 1990 Dec 31;76(6):555–558. doi: 10.1177/030089169007600608. [DOI] [PubMed] [Google Scholar]
- Dawson A. E., Norton J. A., Weinberg D. S. Comparative assessment of proliferation and DNA content in breast carcinoma by image analysis and flow cytometry. Am J Pathol. 1990 May;136(5):1115–1124. [PMC free article] [PubMed] [Google Scholar]
- Derenzini M., Betts C. M., Trerè D., Mambelli V., Millis R. R., Eusebi V., Cancellieri A. Diagnostic value of silver-stained interphasic nucleolar organizer regions in breast tumors. Ultrastruct Pathol. 1990 May-Jun;14(3):233–245. doi: 10.3109/01913129009076127. [DOI] [PubMed] [Google Scholar]
- Donhuijsen K., Schmidt U., Hirche H., van Beuningen D., Budach V. Changes in mitotic rate and cell cycle fractions caused by delayed fixation. Hum Pathol. 1990 Jul;21(7):709–714. doi: 10.1016/0046-8177(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Elston C. W., Ellis I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991 Nov;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Eskelinen M. J., Lipponen P. K., Collan Y., Syrjänen K. J. The role of nucleolar organiser regions as prognostic factors in breast cancer. Eur J Cancer. 1991;27(8):989–992. doi: 10.1016/0277-5379(91)90265-f. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Lippman M. E., Thompson E. B., McGuire W. L., Wittliff J. L., De Sombre E. R., Jensen E. V., Singhakowinta A., Brooks S. C., Jr, Neifeld J. P. Clinical correlations of steroid receptors and male breast cancer. Cancer Res. 1980 Apr;40(4):991–997. [PubMed] [Google Scholar]
- Friedman M. A., Hoffman P. G., Jr, Dandolos E. M., Lagios M. D., Johnston W. H., Siiteri P. K. Estrogen receptors in male breast cancer: clinical and pathologic correlations. Cancer. 1981 Jan 1;47(1):134–137. doi: 10.1002/1097-0142(19810101)47:1<134::aid-cncr2820470122>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Frierson H. F., Jr Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) in infiltrating ductal carcinomas: comparison with clinical and pathologic variables. Mod Pathol. 1993 May;6(3):290–294. [PubMed] [Google Scholar]
- Gasparini G., Meli S., Pozza F., Cazzavillan S., Bevilacqua P. PC-10 antibody to proliferating cell nuclear antigen (PCNA) is not related to prognosis in human breast carcinoma. Growth Regul. 1992 Dec;2(4):145–150. [PubMed] [Google Scholar]
- Giri D. D., Nottingham J. F., Lawry J., Dundas S. A., Underwood J. C. Silver-binding nucleolar organizer regions (AgNORs) in benign and malignant breast lesions: correlations with ploidy and growth phase by DNA flow cytometry. J Pathol. 1989 Apr;157(4):307–313. doi: 10.1002/path.1711570407. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hiort O., Kwan P. W., DeLellis R. A. Immunohistochemistry of estrogen receptor protein in paraffin sections. Effects of enzymatic pretreatment and cobalt chloride intensification. Am J Clin Pathol. 1988 Nov;90(5):559–563. doi: 10.1093/ajcp/90.5.559. [DOI] [PubMed] [Google Scholar]
- Kinne D. W., Ashikari R., Butler A., Menendez-Botet C., Rosen P. P., Schwartz M. Estrogen receptor protein in breast cancer as a predictor of recurrence. Cancer. 1981 May 15;47(10):2364–2367. doi: 10.1002/1097-0142(19810515)47:10<2364::aid-cncr2820471007>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K., Eskelinen M. J. Cell proliferation of transitional cell bladder tumours determined by PCNA/cyclin immunostaining and its prognostic value. Br J Cancer. 1992 Jul;66(1):171–176. doi: 10.1038/bjc.1992.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno W. B., Hirschfield L., Bhuiya T., Harrison G., Mir R. Correlation of proliferative index (PCNA reactivity and Ki-67 reactivity) in primary breast carcinoma with hormone status, lymph node status, and disease-free survival. Conn Med. 1992 Dec;56(12):667–669. [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Clark G. M. Role of progesterone receptors in breast cancer. Semin Oncol. 1985 Mar;12(1 Suppl 1):12–16. [PubMed] [Google Scholar]
- Mourad W. A., Erkman-Balis B., Livingston S., Shoukri M., Cox C. E., Nicosia S. V., Rowlands D. T., Jr Argyrophilic nucleolar organizer regions in breast carcinoma. Correlation with DNA flow cytometry, histopathology, and lymph node status. Cancer. 1992 Apr 1;69(7):1739–1744. doi: 10.1002/1097-0142(19920401)69:7<1739::aid-cncr2820690715>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Nairn E. R., Crocker J., McGovern J. Limited value of AgNOR enumeration in assessment of thyroid neoplasms. J Clin Pathol. 1988 Oct;41(10):1136–1136. doi: 10.1136/jcp.41.10.1136-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Funahashi H., Satoh Y., Takagi H. Proliferating cell nuclear antigen immunostaining in breast cancer and its relation to prognosis. Jpn J Clin Oncol. 1993 Feb;23(1):20–25. [PubMed] [Google Scholar]
- Norris H. J., Taylor H. B. Carcinoma of the male breast. Cancer. 1969 Jun;23(6):1428–1435. doi: 10.1002/1097-0142(196906)23:6<1428::aid-cncr2820230626>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ohri A. K., Ohri S. K., Herbert A., Royle G., Taylor I. The relationship between clinical staging, oestrogen receptor status and silver-binding nucleolar organiser regions (AgNOR) in breast carcinoma. Eur J Surg Oncol. 1992 Apr;18(2):103–107. [PubMed] [Google Scholar]
- Pertschuk L. P., Kim D. S., Nayer K., Feldman J. G., Eisenberg K. B., Carter A. C., Rong Z. T., Thelmo W. L., Fleisher J., Greene G. L. Immunocytochemical estrogen and progestin receptor assays in breast cancer with monoclonal antibodies. Histopathologic, demographic, and biochemical correlations and relationship to endocrine response and survival. Cancer. 1990 Oct 15;66(8):1663–1670. doi: 10.1002/1097-0142(19901015)66:8<1663::aid-cncr2820660802>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pich A., Chiusa L., Pisani P., Krengli M., Pia F., Navone R. Argyrophilic nucleolar organizer region counts and proliferating cell nuclear antigen scores are two reliable indicators of survival in pharyngeal carcinoma. J Cancer Res Clin Oncol. 1992;119(2):106–110. doi: 10.1007/BF01209665. [DOI] [PubMed] [Google Scholar]
- Pich A., Pisani P., Kzengli M., Cappello N., Navone R. Argyrophilic nucleolar organiser region counts and prognosis in pharyngeal carcinoma. Br J Cancer. 1991 Aug;64(2):327–332. doi: 10.1038/bjc.1991.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich A., Ponti R., Valente G., Chiusa L., Geuna M., Novero D., Palestro G. MIB-1, Ki67, and PCNA scores and DNA flow cytometry in intermediate grade malignant lymphomas. J Clin Pathol. 1994 Jan;47(1):18–22. doi: 10.1136/jcp.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploton D., Menager M., Jeannesson P., Himber G., Pigeon F., Adnet J. J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986 Jan;18(1):5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Ribeiro G. Male breast carcinoma--a review of 301 cases from the Christie Hospital & Holt Radium Institute, Manchester. Br J Cancer. 1985 Jan;51(1):115–119. doi: 10.1038/bjc.1985.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Day C. A., Fox S. B. Expression of cathepsin D and estrogen receptor in male breast carcinoma. Hum Pathol. 1993 Feb;24(2):148–151. doi: 10.1016/0046-8177(93)90293-p. [DOI] [PubMed] [Google Scholar]
- Sacks N. P., Robertson J. F., Ellis I. O., Nicholson R. I., Crocker J., Blamey R. W. Silver-stained nucleolar organiser region counts are of no prognostic value in primary breast cancer. Eur J Surg Oncol. 1992 Apr;18(2):98–102. [PubMed] [Google Scholar]
- Sasano H., Saito Y., Sato I., Sasano N., Nagura H. Nucleolar organizer regions in human adrenocortical disorders. Mod Pathol. 1990 Sep;3(5):591–595. [PubMed] [Google Scholar]
- Scheike O. Male breast cancer. 6. Factors influencing prognosis. Br J Cancer. 1974 Sep;30(3):261–271. doi: 10.1038/bjc.1974.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siitonen S. M., Isola J. J., Rantala I. S., Helin H. J. Intratumor variation in cell proliferation in breast carcinoma as determined by antiproliferating cell nuclear antigen monoclonal antibody and automated image analysis. Am J Clin Pathol. 1993 Mar;99(3):226–231. doi: 10.1093/ajcp/99.3.226. [DOI] [PubMed] [Google Scholar]
- Siitonen S. M., Kallioniemi O. P., Isola J. J. Proliferating cell nuclear antigen immunohistochemistry using monoclonal antibody 19A2 and a new antigen retrieval technique has prognostic impact in archival paraffin-embedded node-negative breast cancer. Am J Pathol. 1993 Apr;142(4):1081–1089. [PMC free article] [PubMed] [Google Scholar]
- Sivridis E., Sims B. Nucleolar organiser regions: new prognostic variable in breast carcinomas. J Clin Pathol. 1990 May;43(5):390–392. doi: 10.1136/jcp.43.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toikkanen S., Joensuu H. AgNOR counts have no prognostic value in breast cancer. J Pathol. 1993 Feb;169(2):251–254. doi: 10.1002/path.1711690212. [DOI] [PubMed] [Google Scholar]
- Woods A. L., Hall P. A., Shepherd N. A., Hanby A. M., Waseem N. H., Lane D. P., Levison D. A. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathology. 1991 Jul;19(1):21–27. doi: 10.1111/j.1365-2559.1991.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Yu C. C., Hall P. A., Fletcher C. D., Camplejohn R. S., Waseem N. H., Lane D. P., Levison D. A. Haemangiopericytomas: the prognostic value of immunohistochemical staining with a monoclonal antibody to proliferating cell nuclear antigen (PCNA). Histopathology. 1991 Jul;19(1):29–33. doi: 10.1111/j.1365-2559.1991.tb00891.x. [DOI] [PubMed] [Google Scholar]
- de Capoa A., Baldini A., Marlekaj P., Natoli C., Rocchi M., Archidiacono N., Cianfarani S., Spadoni G. L., Boscherini B. Hormone-modulated rRNA gene activity is visualized by selective staining of the NOs. Cell Biol Int Rep. 1985 Sep;9(9):791–796. doi: 10.1016/0309-1651(85)90097-9. [DOI] [PubMed] [Google Scholar]