Abstract
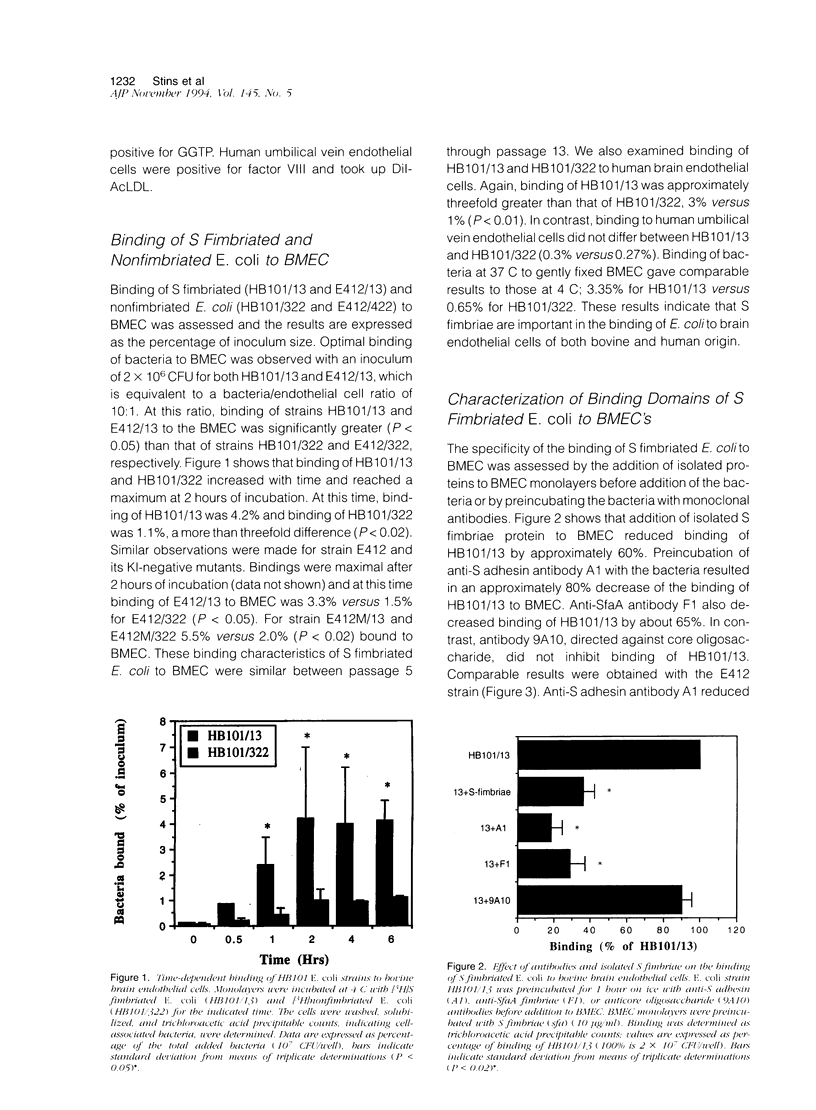
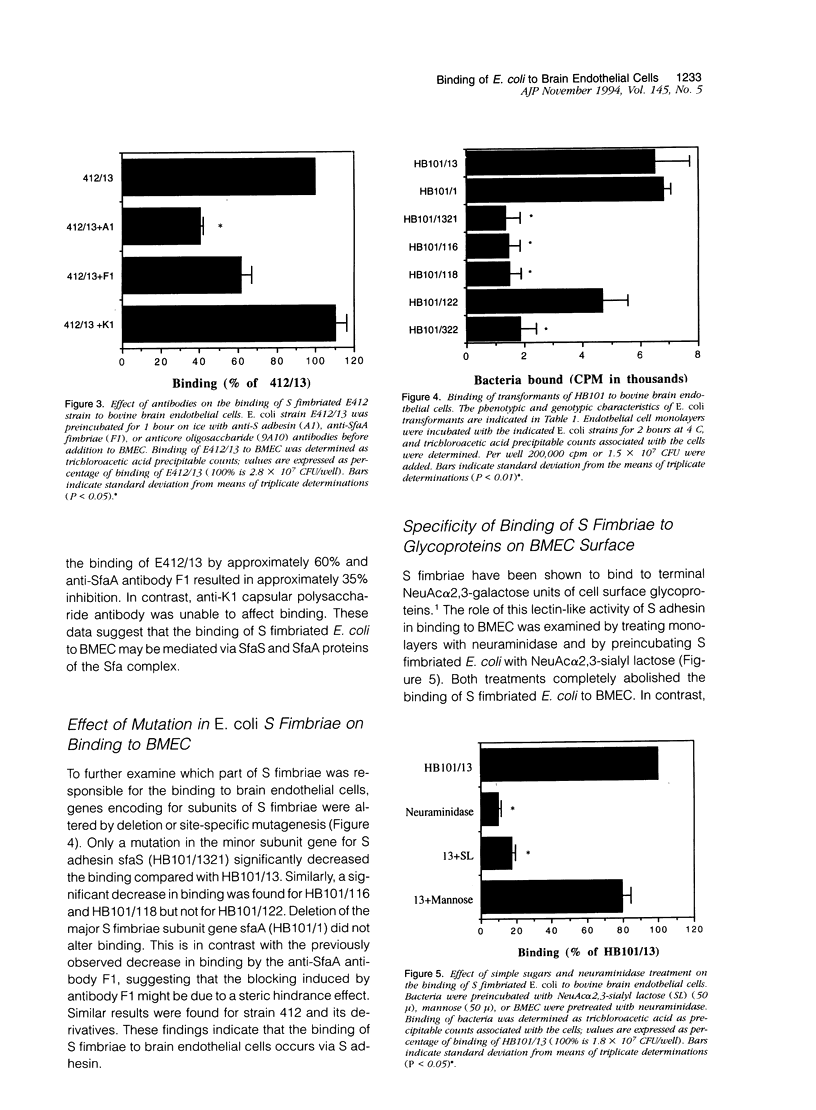
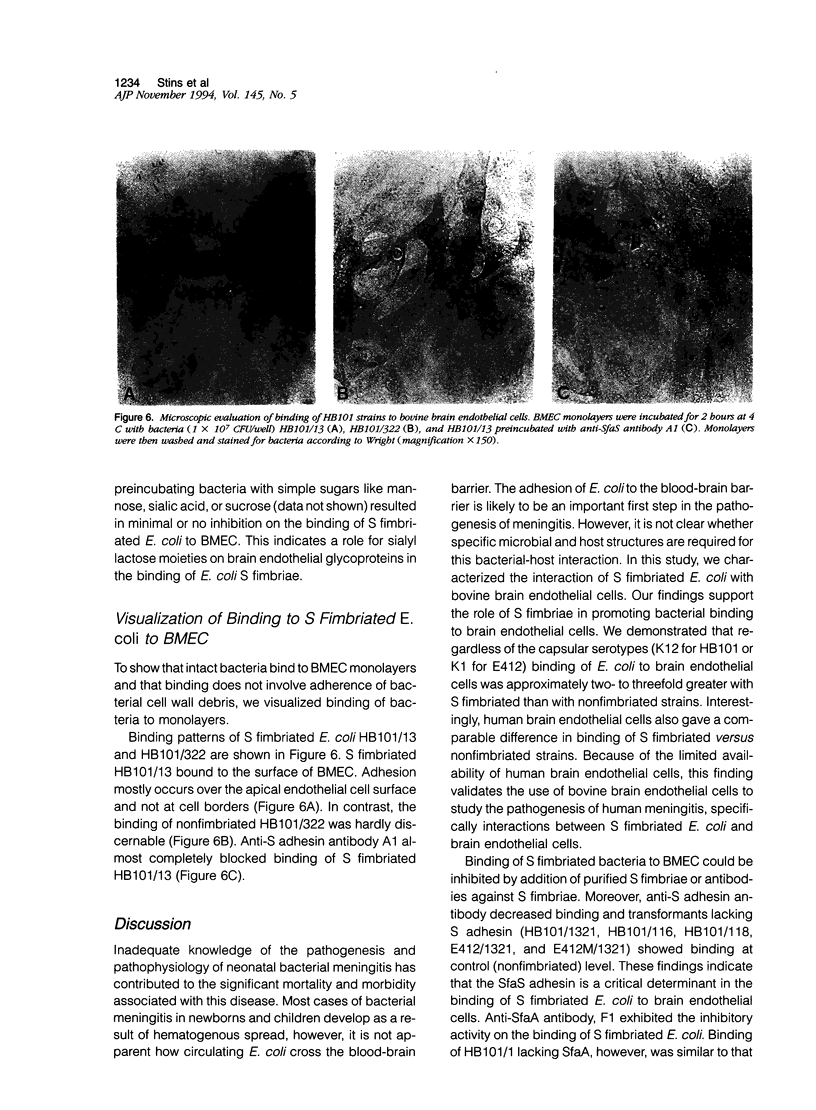
To assess the role of S fimbriae in the pathogenesis of Escherichia coli meningitis, transformants of E. coli strains with or without S fimbriae plasmid were compared for their binding to microvessel endothelial cells isolated from bovine brain cortices (BMEC). The BMEC's displayed a cobblestone appearance, were positive for factor VIII, carbonic anhydrase IV, took up fluorescent-labeled acetylated low density lipoprotein, and exhibited gamma glutamyl transpeptidase activity. Binding of S fimbriated E. coli to BMEC was approximately threefold greater than nonfimbriated E. coli Similarly S fimbriated E. coli bound to human brain endothelial cells approximately threefold greater than nonfimbriated E. coli. Binding was reduced approximately 60% by isolated S fimbriae and about 80% by anti-S adhesin antibody. Mutating the S adhesin gene resulted in a complete loss of the binding, whereas mutagenesis of the major S fimbriae subunit gene sfaA did not significantly affect binding. Pretreatment of BMEC with neuraminidase or prior incubation of S fimbriated E. coli with NeuAc alpha 2,3-sialyl lactose completely abolished binding. These findings indicate that S fimbriated E. coli bind to NeuAc alpha 2,3-galactose containing glycoproteins on brain endothelial cells via a lectin-like activity of SfaS adhesin. This might be an important early step in the penetration of bacteria across the blood-brain barrier in the development of E. coli meningitis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman P. D., Betz A. L., Ar D., Wolinsky J. S., Penney J. B., Shivers R. R., Goldstein G. W. Primary culture of capillary endothelium from rat brain. In Vitro. 1981 Apr;17(4):353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Cancilla P. A. gamma-Glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science. 1980 Feb 8;207(4431):653–655. doi: 10.1126/science.6101511. [DOI] [PubMed] [Google Scholar]
- Ghandour M. S., Langley O. K., Zhu X. L., Waheed A., Sly W. S. Carbonic anhydrase IV on brain capillary endothelial cells: a marker associated with the blood-brain barrier. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6823–6827. doi: 10.1073/pnas.89.15.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. F., Migeon B. R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 1975 May;5(1):11–17. doi: 10.1016/0092-8674(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Gladstone I. M., Ehrenkranz R. A., Edberg S. C., Baltimore R. S. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr Infect Dis J. 1990 Nov;9(11):819–825. doi: 10.1097/00006454-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Wolinsky J. S., Csejtey J., Diamond I. Isolation of metabolically active capillaries from rat brain. J Neurochem. 1975 Nov;25(5):715–717. doi: 10.1111/j.1471-4159.1975.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Hacker J. Genetic determinants coding for fimbriae and adhesins of extraintestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:1–27. doi: 10.1007/978-3-642-74703-8_1. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Cross A. S., Zollinger W., Sadoff J. Prevention and therapy of experimental Escherichia coli infection with monoclonal antibody. Infect Immun. 1985 Dec;50(3):734–737. doi: 10.1128/iai.50.3.734-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Itabashi H., Gemski P., Sadoff J., Warren R. L., Cross A. S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Invest. 1992 Sep;90(3):897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. O., Feigin R. D., McCracken G. H., Jr Report of the Task Force on Diagnosis and Management of Meningitis. Pediatrics. 1986 Nov;78(5 Pt 2):959–982. [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischeck U., Meyer J., Galla H. J. Characterization of gamma-glutamyl transpeptidase activity of cultured endothelial cells from porcine brain capillaries. Cell Tissue Res. 1989 Apr;256(1):221–226. doi: 10.1007/BF00224737. [DOI] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhäuser J., Hoschützky H., Jann K., Hacker J. Functional analysis of the sialic acid-binding adhesin SfaS of pathogenic Escherichia coli by site-specific mutagenesis. Infect Immun. 1990 Jul;58(7):2133–2138. doi: 10.1128/iai.58.7.2133-2138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sessa G., Green J. P. Gamma-glutamyl transpeptidase in brain capillaries: possible site of a blood-brain barrier for amino acids. Science. 1974 Apr 5;184(4132):66–68. doi: 10.1126/science.184.4132.66. [DOI] [PubMed] [Google Scholar]
- Ott M., Hacker J., Schmoll T., Jarchau T., Korhonen T. K., Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986 Dec;54(3):646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Korhonen T. K., Pere A., Hacker J., Soinila S. Binding sites in the rat brain for Escherichia coli S fimbriae associated with neonatal meningitis. J Clin Invest. 1988 Mar;81(3):860–865. doi: 10.1172/JCI113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Prasadarao N. V., Wass C. A., Hacker J., Jann K., Kim K. S. Adhesion of S-fimbriated Escherichia coli to brain glycolipids mediated by sfaA gene-encoded protein of S-fimbriae. J Biol Chem. 1993 May 15;268(14):10356–10363. [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Schmoll T., Morschhäuser J., Ott M., Ludwig B., van Die I., Hacker J. Complete genetic organization and functional aspects of the Escherichia coli S fimbrial adhesion determinant: nucleotide sequence of the genes sfa B, C, D, E, F. Microb Pathog. 1990 Nov;9(5):331–343. doi: 10.1016/0882-4010(90)90067-z. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Korhonen T. K., Väisänen-Rhen V., Williams P. H., Pattison P. E., Caugant D. A. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986 Apr;52(1):213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Gillis J. F., Matthews M. A., Wagner R. C., Bitensky M. W. Isolation and characterization of brain endothelial cells: morphology and enzyme activity. J Neurochem. 1980 Aug;35(2):374–381. doi: 10.1111/j.1471-4159.1980.tb06274.x. [DOI] [PubMed] [Google Scholar]



