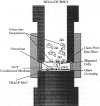
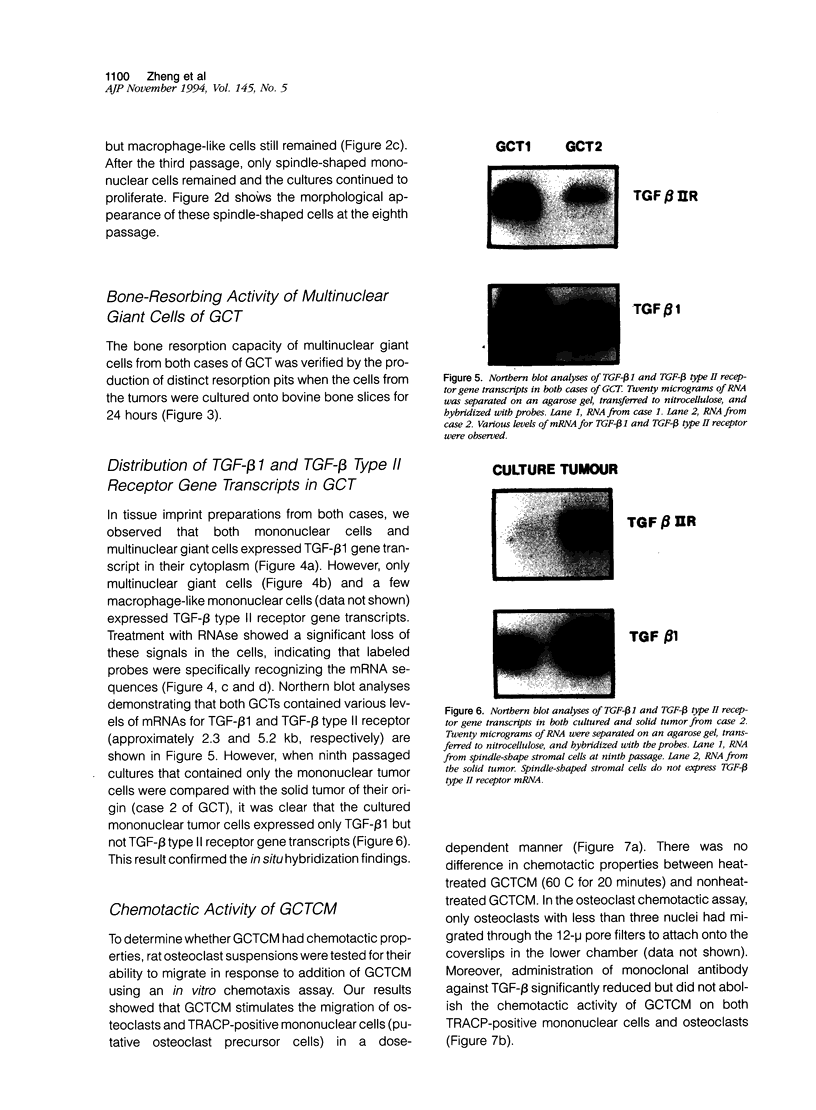
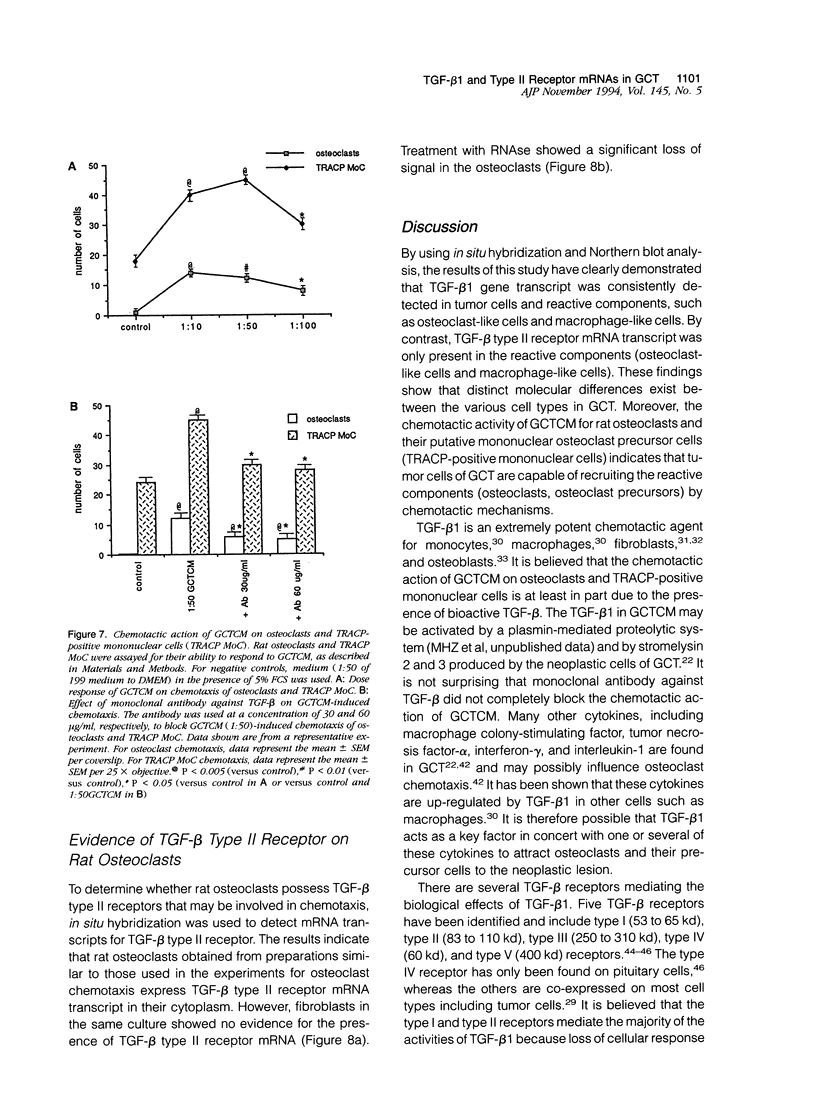
Abstract
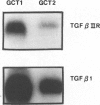
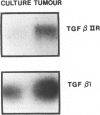
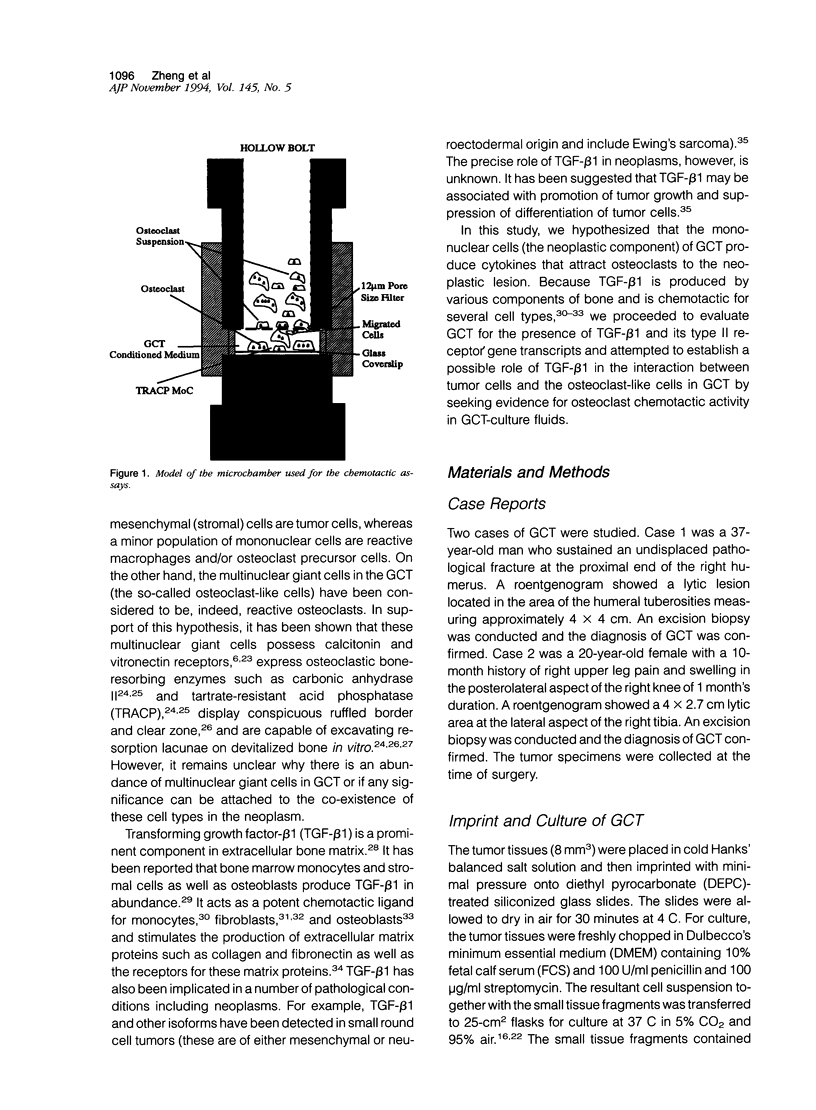
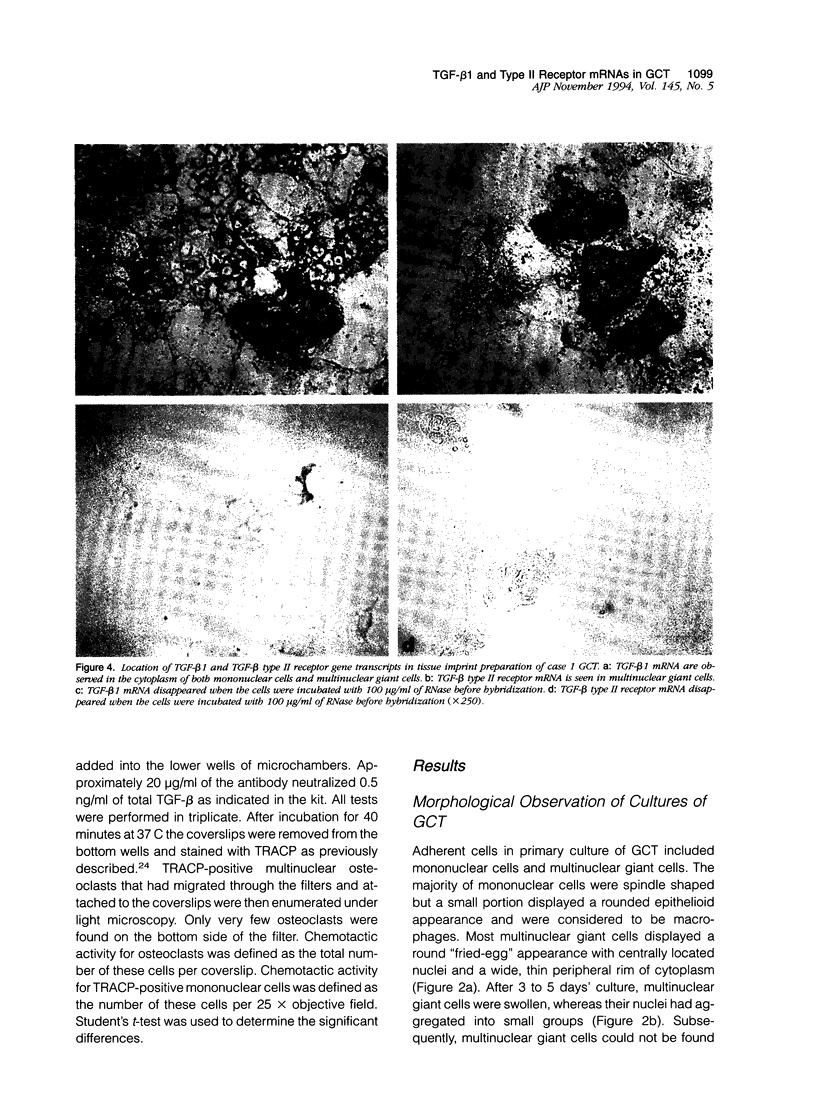
Giant cell tumor of bone (GCT) is a relatively rare skeletal neoplasm characterized by multinuclear giant cells (osteoclast-like cells) scattered in a mass of mononuclear cells. The currently favored hypothesis for the origin of cells within GCT is that the multinuclear giant cells are reactive osteoclasts, whereas the truly neoplastic cells are the major component of the mononuclear population. However, the pathological significance and the precise relationship of tumor cells and osteoclast-like cells in GCT have not been fully established. In this study, we evaluated two GCTs for the presence of transforming growth factor-beta 1 (TGF-beta 1) and TGF-beta type II receptor gene transcripts and attempted to establish a possible role for TGF-beta 1 in the interaction between tumor cells and osteoclast-like cells. By using in situ hybridization and Northern blot analysis, we have demonstrated that TGF-beta 1 mRNA transcript is consistently detected in both tumor mononuclear cells and osteoclast-like cells, whereas TGF-beta type II receptor gene transcript is only present in osteoclast-like cells. Moreover, isolated rat osteoclasts were tested for their ability to migrate in response to GCT-conditioned medium (GCTCM) in an in vitro chemotactic assay. Our results showed that GCTCM stimulates the migration of osteoclasts in a dose-dependent manner. Interestingly, only osteoclasts containing less than three nuclei can migrate through 12-mu pore filters. Addition of monoclonal antibody against TGF-beta significantly reduced but did not abolish the chemotactic activity of GCTCM. Moreover, TGF-beta type II receptor mRNA has been demonstrated in the normal rat osteoclasts and may be involved in the chemotactic action of TGF-beta 1. We concluded that TGF-beta 1, possibly in concert with other cytokines, is involved in the recruitment of osteoclast-like cells in GCT by acting in an autocrine or paracrine fashion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanasou N. A., Bliss E., Gatter K. C., Heryet A., Woods C. G., McGee J. O. An immunohistological study of giant-cell tumour of bone: evidence for an osteoclast origin of the giant cells. J Pathol. 1985 Nov;147(3):153–158. doi: 10.1002/path.1711470302. [DOI] [PubMed] [Google Scholar]
- Bouropoulou V., Kontogeorgos G., Manika Z. A histological and immunoenzymatic study on the histogenesis of "giant cell tumor of bones". Pathol Res Pract. 1985 Jul;180(1):61–67. doi: 10.1016/S0344-0338(85)80076-5. [DOI] [PubMed] [Google Scholar]
- Brecher M. E., Franklin W. A., Simon M. A. Immunohistochemical study of mononuclear phagocyte antigens in giant cell tumor of bone. Am J Pathol. 1986 Nov;125(2):252–257. [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Winchester R. J., Dimitriu-Bona A., Klein M., Steiner G., Sissons H. A. Delineation of four cell types comprising the giant cell tumor of bone. Expression of Ia and monocyte-macrophage lineage antigens. J Clin Invest. 1983 Jun;71(6):1633–1648. doi: 10.1172/JCI110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Fuller K., McSheehy P. M., Pringle J. A. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol. 1985 Apr;145(4):297–305. doi: 10.1002/path.1711450403. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Ling N., Guillemin R., Massagué J. A surface component on GH3 pituitary cells that recognizes transforming growth factor-beta, activin, and inhibin. J Biol Chem. 1988 Nov 25;263(33):17225–17228. [PubMed] [Google Scholar]
- Chen R. H., Ebner R., Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-beta activities. Science. 1993 May 28;260(5112):1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Dahlin D. C., Cupps R. E., Johnson E. W., Jr Giant-cell tumor: a study of 195 cases. Cancer. 1970 May;25(5):1061–1070. doi: 10.1002/1097-0142(197005)25:5<1061::aid-cncr2820250509>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Emura I., Inoue Y., Ohnishi Y., Morita T., Saito H., Tajima T. Histochemical, immunohistochemical and ultrastructural investigations of giant cell tumors of bone. Acta Pathol Jpn. 1986 May;36(5):691–702. doi: 10.1111/j.1440-1827.1986.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Petrison K. K., Bhan A. K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987 Feb;79(2):483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S. R., Schiller A. L., Mankin H. J., Dayer J. M., Krane S. M. Characterization of cells from human giant cell tumors of bone. Clin Orthop Relat Res. 1986 Mar;(204):59–75. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Generation of osteoclasts from hemopoietic cells and a multipotential cell line in vitro. J Cell Physiol. 1989 Sep;140(3):478–482. doi: 10.1002/jcp.1041400311. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Pringle J. A., Chambers T. J. Identification of human osteoclasts with monoclonal antibodies. N Engl J Med. 1985 Apr 4;312(14):923–924. doi: 10.1056/NEJM198504043121418. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Rimmer E. F., Lewis D., Pringle J. A., Fuller K., Chambers T. J. Cell surface characterization of the human osteoclast: phenotypic relationship to other bone marrow-derived cell types. J Pathol. 1984 Dec;144(4):281–294. doi: 10.1002/path.1711440410. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Green A. D., Beattie C. W., Das Gupta T. K. Monocyte-macrophage lineage of giant cell tumor of bone. Establishment of a multinucleated cell line. Cancer. 1993 Mar 1;71(5):1751–1760. doi: 10.1002/1097-0142(19930301)71:5<1751::aid-cncr2820710509>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Joyner C. J., Quinn J. M., Triffitt J. T., Owen M. E., Athanasou N. A. Phenotypic characterisation of mononuclear and multinucleated cells of giant cell tumour of bone. Bone Miner. 1992 Jan;16(1):37–48. doi: 10.1016/0169-6009(92)90820-4. [DOI] [PubMed] [Google Scholar]
- Kanehisa J., Izumo T., Takeuchi M., Yamanaka T., Fujii T., Takeuchi H. In vitro bone resorption by isolated multinucleated giant cells from giant cell tumour of bone: light and electron microscopic study. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):327–338. doi: 10.1007/BF01606524. [DOI] [PubMed] [Google Scholar]
- Kito M., Moriya H., Mikata A., Harigaya K., Takenouchi T., Takada N., Tatezaki S., Umeda T. Establishment of a cell line from a human giant cell tumor of bone. Clin Orthop Relat Res. 1993 Sep;(294):353–360. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Ling L., Klein M. J., Sissons H. A., Steiner G. C., Winchester R. J. Expression of Ia and monocyte-macrophage lineage antigens in giant cell tumor of bone and related lesions. Arch Pathol Lab Med. 1988 Jan;112(1):65–69. [PubMed] [Google Scholar]
- Liu T. C., Ji Z. M., Wang L. T. Giant cell tumors of bone. An immunohistochemical study. Pathol Res Pract. 1989 Oct;185(4):448–453. [PubMed] [Google Scholar]
- Lucas P. A., Caplan A. I. Chemotactic response of embryonic limb bud mesenchymal cells and muscle-derived fibroblasts to transforming growth factor-beta. Connect Tissue Res. 1988;18(1):1–7. doi: 10.3109/03008208809019068. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- McCune B. K., Patterson K., Chandra R. S., Kapur S., Sporn M. B., Tsokos M. Expression of transforming growth factor-beta isoforms in small round cell tumors of childhood. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- O'Grady P., Kuo M. D., Baldassare J. J., Huang S. S., Huang J. S. Purification of a new type high molecular weight receptor (type V receptor) of transforming growth factor beta (TGF-beta) from bovine liver. Identification of the type V TGF-beta receptor in cultured cells. J Biol Chem. 1991 May 5;266(13):8583–8589. [PubMed] [Google Scholar]
- Pfeilschifter J., Wolf O., Naumann A., Minne H. W., Mundy G. R., Ziegler R. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J Bone Miner Res. 1990 Aug;5(8):825–830. doi: 10.1002/jbmr.5650050805. [DOI] [PubMed] [Google Scholar]
- Piper K., Boyde A., Jones S. J. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat Embryol (Berl) 1992 Sep;186(4):291–299. doi: 10.1007/BF00185977. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T. A., Maley M. A., Grounds M. D., Papadimitriou J. M. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993 Aug;207(2):321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- Roessner A., von Bassewitz D. B., Schlake W., Thorwesten G., Grundmann E. Biologic characterization of human bone tumors. III. Giant cell tumor of bone. A combined electron microscopical, histochemical, and autoradiographical study. Pathol Res Pract. 1984 May;178(5):431–440. doi: 10.1016/s0344-0338(84)80002-3. [DOI] [PubMed] [Google Scholar]
- Sasaguri Y., Komiya S., Sugama K., Suzuki K., Inoue A., Morimatsu M., Nagase H. Production of matrix metalloproteinases 2 and 3 (stromelysin) by stromal cells of giant cell tumor of bone. Am J Pathol. 1992 Sep;141(3):611–621. [PMC free article] [PubMed] [Google Scholar]
- Steiner G. C., Ghosh L., Dorfman H. D. Ultrastructure of giant cell tumors of bone. Hum Pathol. 1972 Dec;3(4):569–586. doi: 10.1016/s0046-8177(72)80007-8. [DOI] [PubMed] [Google Scholar]
- Toyosawa S., Ogawa Y., Chang C. K., Hong S. S., Yagi T., Kuwahara H., Wakasa K., Sakurai M. Histochemistry of tartrate-resistant acid phosphatase and carbonic anhydrase isoenzyme II in osteoclast-like giant cells in bone tumours. Virchows Arch A Pathol Anat Histopathol. 1991;418(3):255–261. doi: 10.1007/BF01606064. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Akeho M., Yumoto T. Giant cell tumor bone. Enzyme histochemical, biochemical and tissue culture studies. Virchows Arch A Pathol Anat Histol. 1982;395(3):319–330. doi: 10.1007/BF00429357. [DOI] [PubMed] [Google Scholar]
- Zheng M. H., Fan Y., Wysocki S., Wood D. J., Papadimitriou J. M. Detection of mRNA for carbonic anhydrase II in human osteoclast-like cells by in situ hybridization. J Bone Miner Res. 1993 Jan;8(1):113–118. doi: 10.1002/jbmr.5650080114. [DOI] [PubMed] [Google Scholar]
- Zheng M. H., Papadimitriou J. M., Nicholson G. C. RNA synthesis in isolated rat osteoclasts: inhibitory effect of calcitonin. Bone. 1991;12(5):317–322. doi: 10.1016/8756-3282(91)90017-d. [DOI] [PubMed] [Google Scholar]
- Zheng M. H., Wood D. J., Papadimitriou J. M. What's new in the role of cytokines on osteoblast proliferation and differentiation? Pathol Res Pract. 1992 Dec;188(8):1104–1121. doi: 10.1016/S0344-0338(11)81263-X. [DOI] [PubMed] [Google Scholar]