Abstract
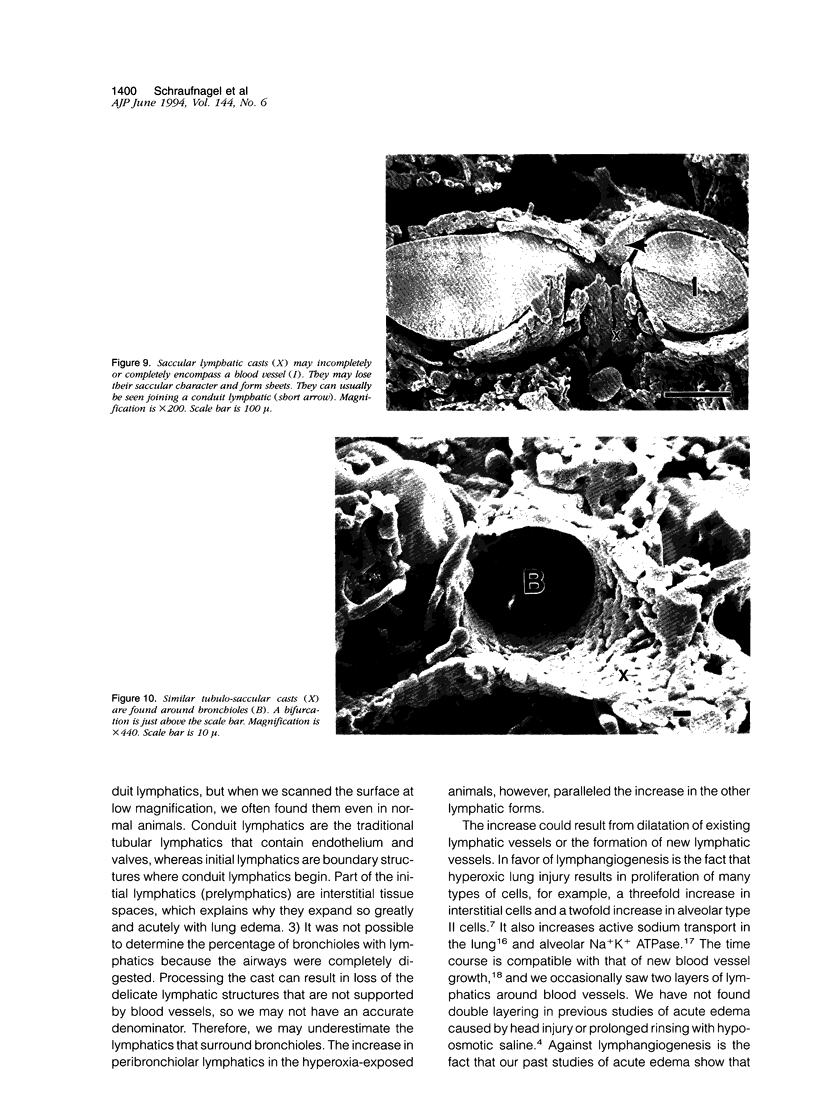
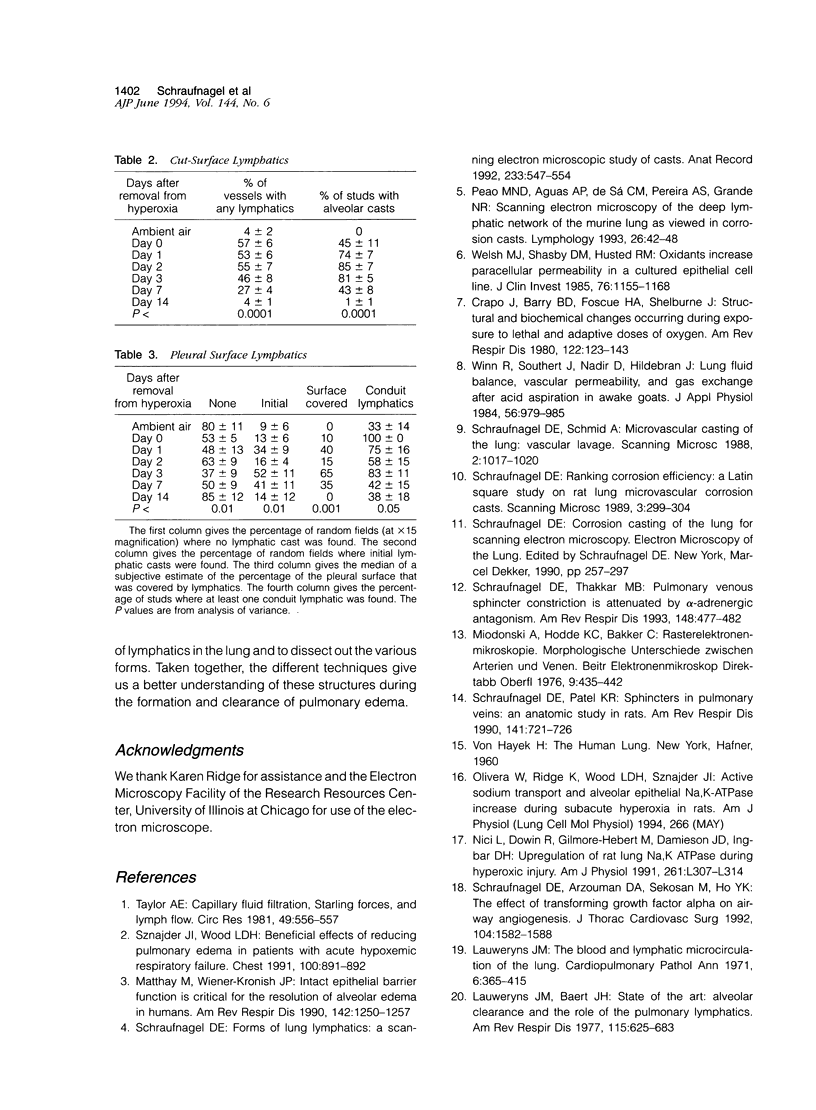
The microscopic lymphatics of the lung can be cast and studied with scanning electron microscopy. This technique shows several different forms of lymphatics and the interstitial space that leads into lymphatics as no other method can. To study changes in lymphatic forms, rats were placed in 85% oxygen for 7 days to produce pulmonary edema. Methyl methacrylate resin was injected into the lung vasculature at various times after the animals were removed from hyperoxia. In the animals not exposed to hyperoxia, no artery, vein, or airway was surrounded by a lymphatic cast. However, in rats that were in the hyperoxic chamber, 22% of arteries, 30% of veins, and 51% of indeterminate blood vessels (which could be arteries or veins) were encompassed by saccular lymphatic casts. These lymphatics were still observed 7 days after recovery from hyperoxia. Fourteen days after hyperoxia, the lymphatics returned to control values. Only 9% of the pleural surface of the animals not exposed to hyperoxia had initial lymphatics. Fifty-two percent of the hyperoxia-exposed animals had initial lymphatics, measured 3 days after exposure. This decreased to 14% 14 days after exposure to hyperoxia (P < 0.01). Conduit lymphatics were found on the pleural surfaces of 33% of animals exposed to ambient air and 100% of animals exposed to the high-oxygen environment (P < 0.05). The median percentage of the pleural surface covered with lymphatics was 0 in the animals exposed to ambient air. It was 65% in animals exposed to hyperoxia, 3 days after returning to room air. It was again 0 in animals exposed to hyperoxia, 14 days after returning to room air (P < 0.001). The lymphatics around veins expanded more than around arteries (P < 0.0001). These results indicate that in the rat all compartments of the lung lymphatics expand after the injury and edema caused by oxygen and return to normal with the resolution of the edema.
Full text
PDF






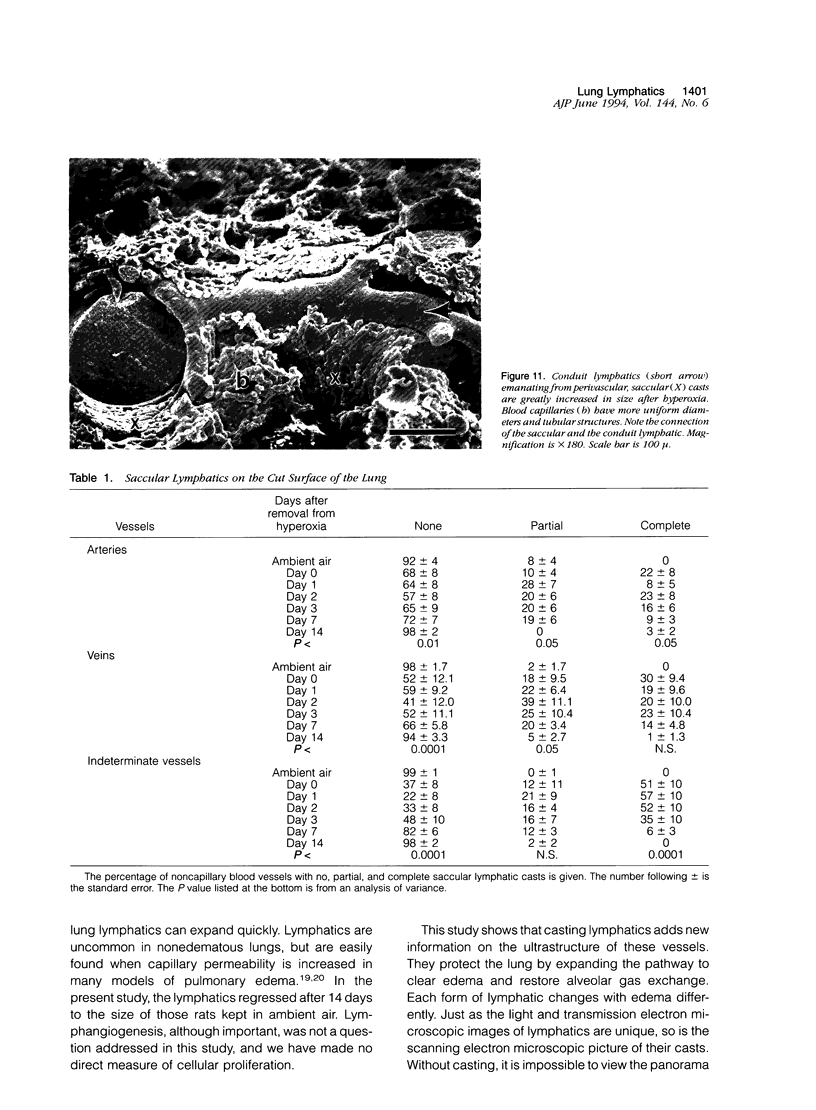
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Baert J. H. Alveolar clearance and the role of the pulmonary lymphatics. Am Rev Respir Dis. 1977 Apr;115(4):625–683. doi: 10.1164/arrd.1977.115.4.625. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M. The blood and lymphatic microcirculation of the lung. Pathol Annu. 1971;6:365–415. [PubMed] [Google Scholar]
- Matthay M. A., Wiener-Kronish J. P. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- Nici L., Dowin R., Gilmore-Hebert M., Jamieson J. D., Ingbar D. H. Upregulation of rat lung Na-K-ATPase during hyperoxic injury. Am J Physiol. 1991 Oct;261(4 Pt 1):L307–L314. doi: 10.1152/ajplung.1991.261.4.L307. [DOI] [PubMed] [Google Scholar]
- Peão M. N., Aguas A. P., de Sá C. M., Pereira A. S., Grande N. R. Scanning electron microscopy of the deep lymphatic network of the murine lung as viewed in corrosion casts. Lymphology. 1993 Mar;26(1):42–48. [PubMed] [Google Scholar]
- Schraufnagel D. E., Arzouman D. A., Sekosan M., Ho Y. K. The effect of transforming growth factor-alpha on airway angiogenesis. J Thorac Cardiovasc Surg. 1992 Dec;104(6):1582–1588. [PubMed] [Google Scholar]
- Schraufnagel D. E. Forms of lung lymphatics: a scanning electron microscopic study of casts. Anat Rec. 1992 Aug;233(4):547–554. doi: 10.1002/ar.1092330409. [DOI] [PubMed] [Google Scholar]
- Schraufnagel D. E., Patel K. R. Sphincters in pulmonary veins. An anatomic study in rats. Am Rev Respir Dis. 1990 Mar;141(3):721–726. doi: 10.1164/ajrccm/141.3.721. [DOI] [PubMed] [Google Scholar]
- Schraufnagel D. E. Ranking corrosion efficiency: a Latin square study on rat lung microvascular corrosion casts. Scanning Microsc. 1989 Mar;3(1):299–304. [PubMed] [Google Scholar]
- Schraufnagel D. E., Schmid A. Microvascular casting of the lung: vascular lavage. Scanning Microsc. 1988 Jun;2(2):1017–1020. [PubMed] [Google Scholar]
- Schraufnagel D. E., Thakkar M. B. Pulmonary venous sphincter constriction is attenuated by alpha-adrenergic antagonism. Am Rev Respir Dis. 1993 Aug;148(2):477–482. doi: 10.1164/ajrccm/148.2.477. [DOI] [PubMed] [Google Scholar]
- Sznajder J. I., Wood L. D. Beneficial effects of reducing pulmonary edema in patients with acute hypoxemic respiratory failure. Chest. 1991 Oct;100(4):890–892. doi: 10.1378/chest.100.4.890. [DOI] [PubMed] [Google Scholar]
- Taylor A. E. Capillary fluid filtration. Starling forces and lymph flow. Circ Res. 1981 Sep;49(3):557–575. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Shasby D. M., Husted R. M. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985 Sep;76(3):1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn R., Stothert J., Nadir B., Hildebrandt J. Lung fluid balance, vascular permeability, and gas exchange after acid aspiration in awake goats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):979–985. doi: 10.1152/jappl.1984.56.4.979. [DOI] [PubMed] [Google Scholar]