Abstract
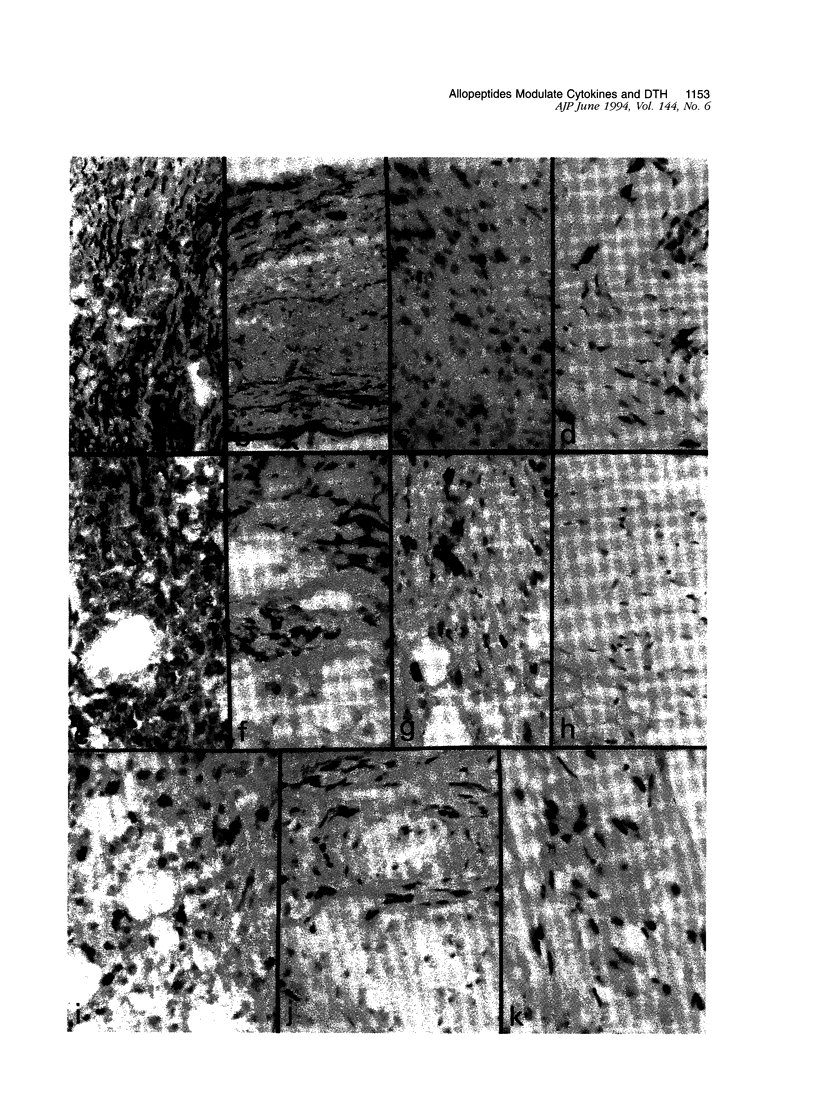
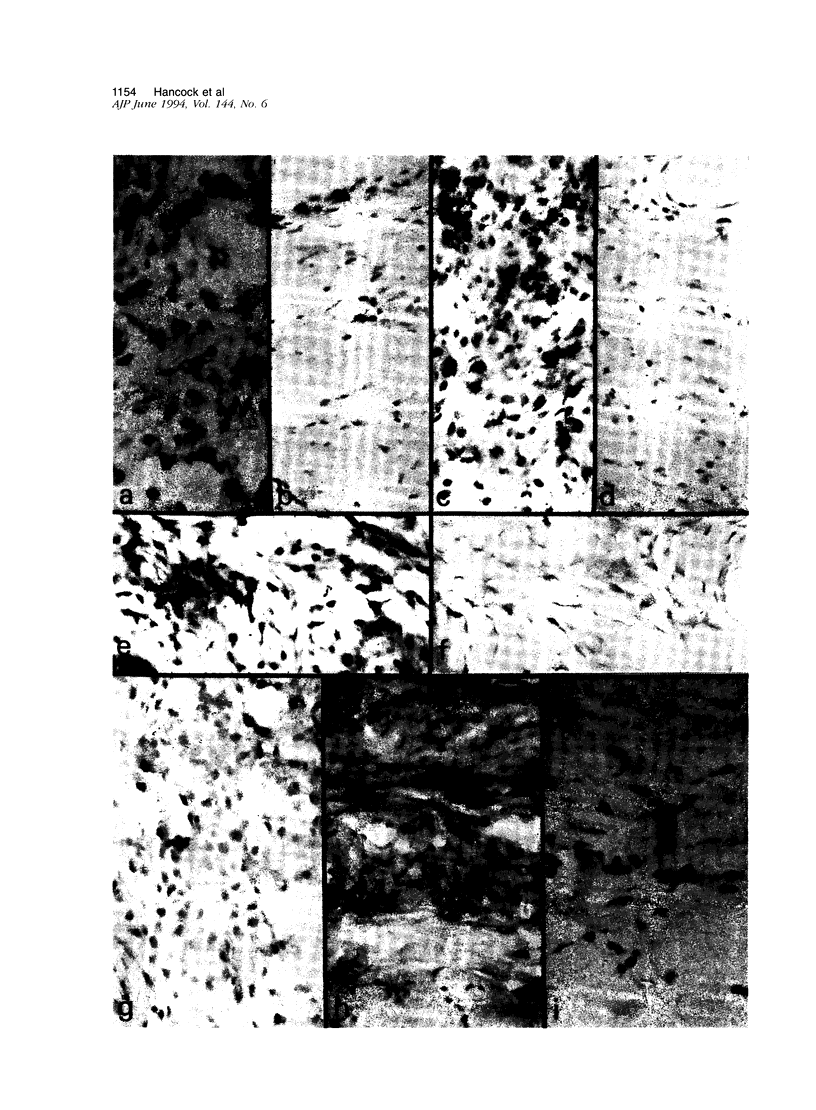
Oral and intrathymic exposure to antigen can each induce systemic antigen-specific immune tolerance, but the mechanisms have not been well defined. We studied the effects that the route of exposure to antigen has on the mechanisms of tolerance in vivo using synthetic polymorphic class II major histocompatibility complex (MHC) peptides in a skin delayed-type hypersensitivity (DTH) response model. Lewis rats were immunized by injection in the footpad with synthetic peptides (RT1.Bu and/or RT1.Du) derived from the hypervariable domain of MHC class II beta chain of the Wistar-Furth rat in complete Freund's adjuvant and challenged 2 weeks later by injection in the ear with the MHC peptides. An "oral" group received the peptide mixture by gavage (100 micrograms/day for 5 days) 3 days before immunization, and an "intrathymic" group received a single intrathymic injection of 100 micrograms of peptides 48 hours before immunization. Oral therapy reduced the DTH response to 23 +/- 7%, and intrathymic exposure reduced the DTH response to 26 +/- 6% (P < 0.001) as compared with control DTH responses of unmodified Lewis animals. Immunohistological evaluation of DTH skin lesions showed that oral and intrathymic therapy each decreased mononuclear cell infiltration, fibrin deposition, and endothelial activation when compared with that seen in control rats. In contrast, while both protocols markedly reduced interleukin (IL-2) and interferon-gamma expression, they had differing effects on local expression of IL-4, transforming growth factor-beta, IL-2R, and CD45RC (a possible discriminant between Th1 and Th2 cells in rats). Oral therapy was associated with increased expression of IL-4 and preservation of transforming growth factor-beta expression by residual IL-2R+, CD45RC- mononuclear and endothelial cells, whereas thymic exposure suppressed essentially all features of immune activation including IL-2R induction and cytokine expression. Our data a) document the detailed pattern of cytokine expression and mononuclear and endothelial cell activation markers during DTH responses and b) confirm that oral tolerance is associated with immune deviation to a predominance of Th2 cell function, whereas intrathymic tolerance may be mediated by T-cell anergy or clonal deletion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus D. B., Surh C. D., Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991 May 1;173(5):1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao N. J., Timmerman L., McDevitt H. O., Jacob C. O. Molecular characterization of MHC class II antigens (beta 1 domain) in the BB diabetes-prone and -resistant rat. Immunogenetics. 1989;29(4):231–234. doi: 10.1007/BF00717906. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M., Johnson R. A., Manseau E. J., Morgan E., Colvin R. B. Morphology of delayed type hypersensitivity reactions in man. I. Quantitative description of the inflammatory response. Lab Invest. 1974 Aug;31(2):111–130. [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M. Morphology of delayed-type hypersensitivity reactions in man. J Invest Dermatol. 1976 Sep;67(3):391–401. doi: 10.1111/1523-1747.ep12514713. [DOI] [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. The role of leukocytes in the activation of blood coagulation. Semin Hematol. 1992 Jul;29(3):202–212. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. M. Cells mediating allograft rejection. Transplantation. 1991 Jun;51(6):1141–1151. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- Hancock W. H., Whitley W. D., Tullius S. G., Heemann U. W., Wasowska B., Baldwin W. M., 3rd, Tilney N. L. Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation. 1993 Sep;56(3):643–650. doi: 10.1097/00007890-199309000-00028. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Sayegh M. H., Kwok C. A., Weiner H. L., Carpenter C. B. Oral, but not intravenous, alloantigen prevents accelerated allograft rejection by selective intragraft Th2 cell activation. Transplantation. 1993 May;55(5):1112–1118. doi: 10.1097/00007890-199305000-00034. [DOI] [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S. J., Sayegh M. H., Hancock W. W., Gallon L., Carpenter C. B., Weiner H. L. Acquired tolerance to experimental autoimmune encephalomyelitis by intrathymic injection of myelin basic protein or its major encephalitogenic peptide. J Exp Med. 1993 Aug 1;178(2):559–566. doi: 10.1084/jem.178.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kradin R. L., Xia W., McCarthy K., Schneeberger E. E. FcR+/- subsets of Ia+ pulmonary dendritic cells in the rat display differences in their abilities to provide accessory co-stimulation for naive (OX-22+) and sensitized (OX-22-) T cells. Am J Pathol. 1993 Mar;142(3):811–819. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Miller A., Zhang Z. J., Sobel R. A., al-Sabbagh A., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993 Jul;46(1-2):73–82. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Ohzato H., Monaco A. P. Induction of specific unresponsiveness (tolerance) to skin allografts by intrathymic donor-specific splenocyte injection in antilymphocyte serum-treated mice. Transplantation. 1992 Dec;54(6):1090–1095. doi: 10.1097/00007890-199212000-00026. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Posselt A. M., Barker C. F., Tomaszewski J. E., Markmann J. F., Choti M. A., Naji A. Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science. 1990 Sep 14;249(4974):1293–1295. doi: 10.1126/science.2119056. [DOI] [PubMed] [Google Scholar]
- Remuzzi G., Rossini M., Imberti O., Perico N. Kidney graft survival in rats without immunosuppressants after intrathymic glomerular transplantation. Lancet. 1991 Mar 30;337(8744):750–752. doi: 10.1016/0140-6736(91)91368-5. [DOI] [PubMed] [Google Scholar]
- Sarawar S. R., Sparshott S. M., Sutton P., Yang C. P., Hutchinson I. V., Bell E. B. Rapid re-expression of CD45RC on rat CD4 T cells in vitro correlates with a change in function. Eur J Immunol. 1993 Jan;23(1):103–109. doi: 10.1002/eji.1830230117. [DOI] [PubMed] [Google Scholar]
- Sayegh M. H., Khoury S. J., Hancock W. W., Weiner H. L., Carpenter C. B. Induction of immunity and oral tolerance with polymorphic class II major histocompatibility complex allopeptides in the rat. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7762–7766. doi: 10.1073/pnas.89.16.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh M. H., Perico N., Imberti O., Hancock W. W., Carpenter C. B., Remuzzi G. Thymic recognition of class II major histocompatibility complex allopeptides induces donor-specific unresponsiveness to renal allografts. Transplantation. 1993 Aug;56(2):461–465. doi: 10.1097/00007890-199308000-00040. [DOI] [PubMed] [Google Scholar]
- Sayegh M. H., Zhang Z. J., Hancock W. W., Kwok C. A., Carpenter C. B., Weiner H. L. Down-regulation of the immune response to histocompatibility antigens and prevention of sensitization by skin allografts by orally administered alloantigen. Transplantation. 1992 Jan;53(1):163–166. doi: 10.1097/00007890-199201000-00033. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tilney N. L., Kupiec-Weglinski J. W. Maturing thymocytes in accelerated rejection of cardiac allografts in presensitized rats. Transplantation. 1992 Sep;54(3):515–519. doi: 10.1097/00007890-199209000-00024. [DOI] [PubMed] [Google Scholar]
- Tipping P. G., Hancock W. W. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- Xu-Amano J., Aicher W. K., Taguchi T., Kiyono H., McGhee J. R. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Int Immunol. 1992 Apr;4(4):433–445. doi: 10.1093/intimm/4.4.433. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992 Aug 15;149(4):1470–1475. [PubMed] [Google Scholar]