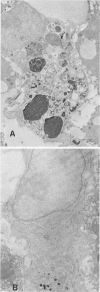
Abstract
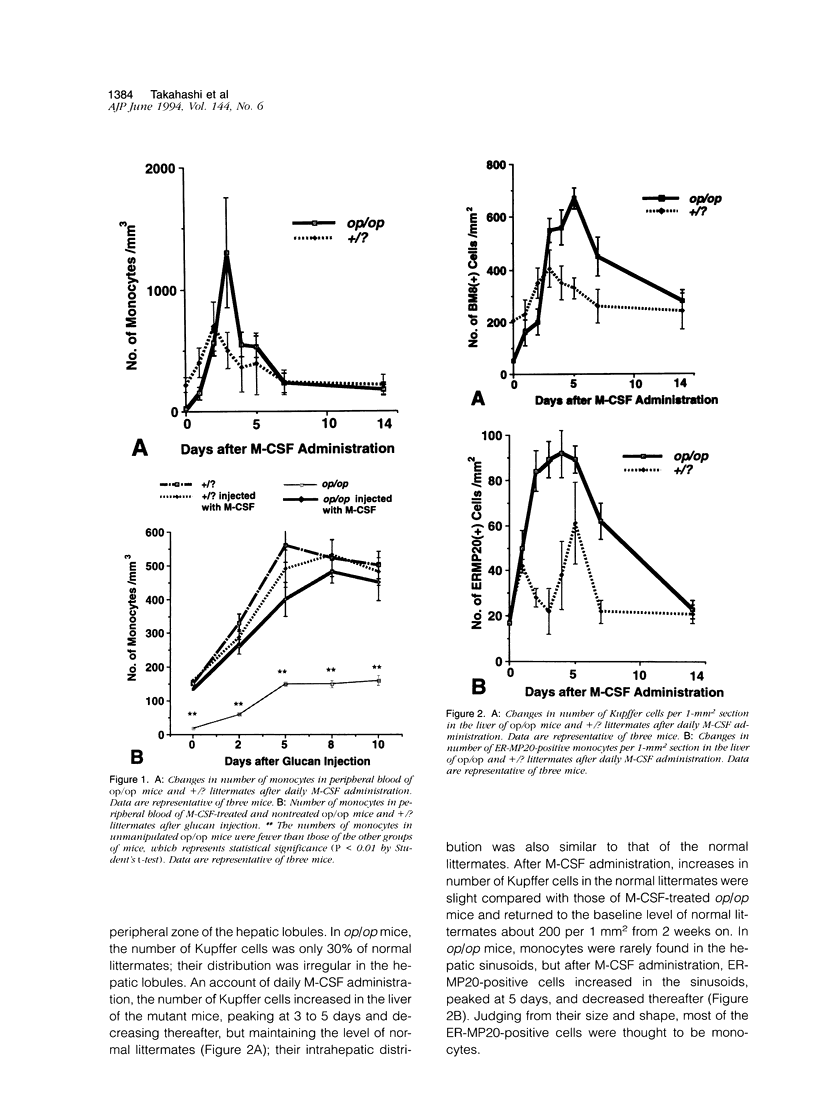
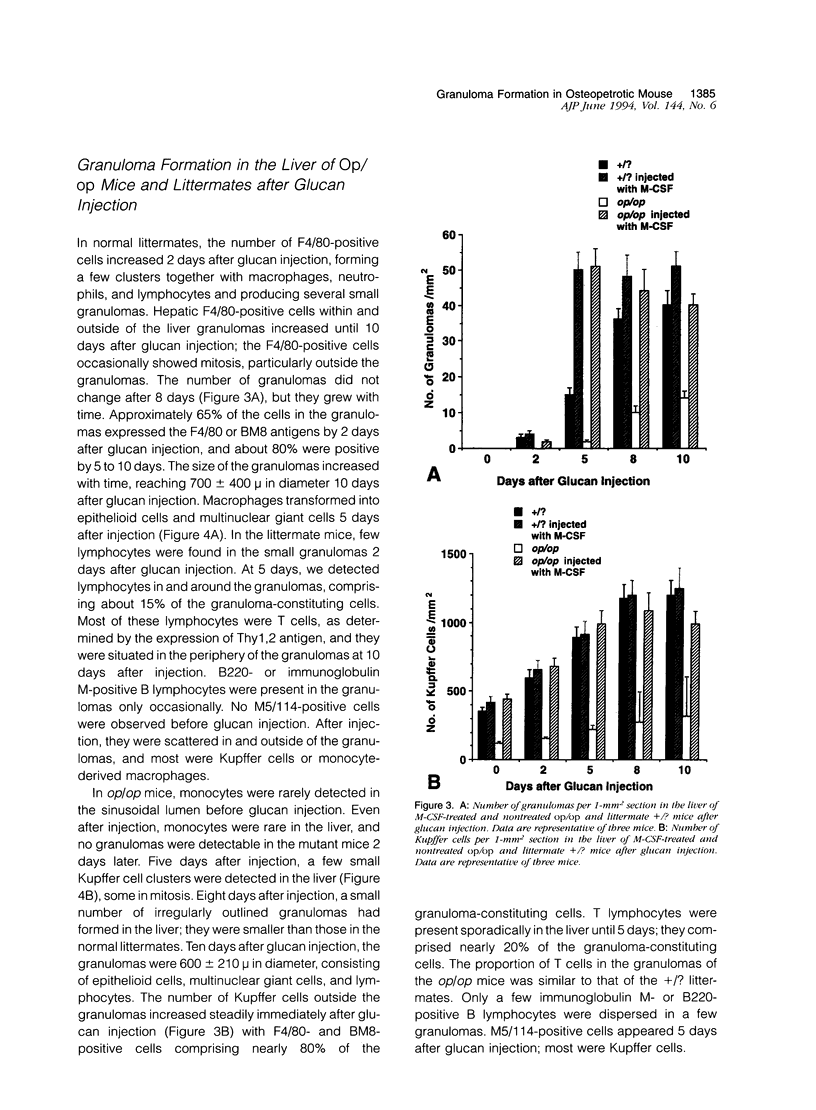
To elucidate the effects of macrophage colony-stimulating factor (M-CSF) on Kupffer cells and monocyte/macrophages in hepatic granuloma formation, we examined granulomas produced by glucan injection in the liver of osteopetrotic mice and littermates with or without M-CSF administration. In the osteopetrotic mice, monocytes were deficient in peripheral blood, and their number did not increase after glucan injection. Hepatic granulomas were formed in the osteopetrotic mice by glucan injection without a supply of blood monocytes. During this process, M-CSF-independent Kupffer cells proliferated, particularly before the granuloma formation, clustered in the hepatic sinusoid, and transformed into epithelioid cells and multinuclear giant cells. In the M-CSF-treated osteopetrotic mice, glucan injection induced an increase in the number of blood monocytes and formed hepatic granulomas at a nearly similar degree to that of littermate mice. Thus, it is concluded that neither monocytes nor M-CSF are necessary for granuloma formation. In contrast, Kupffer cells play a crucial role as granulomas develop in M-CSF-uninjected osteopetrotic mice.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Hepatic granulomas induced by glucan. An ultrastructural and peroxidase-cytochemical study. Lab Invest. 1980 Aug;43(2):172–181. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Induction of focal hemopoiesis in adult rat liver by glucan, a macrophage activator. A cytochemical and ultrastructural study. Lab Invest. 1980 Feb;42(2):217–224. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. The appearance of transition forms between monocytes and Kupffer cells in the liver of rats treated with glucan. A cytochemical and ultrastructural study. J Exp Med. 1979 Apr 1;149(4):883–897. doi: 10.1084/jem.149.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. E., Righthand V. F., Boros D. L. Characterization of regulatory (interferon-alpha/beta) and accessory (LAF/IL 1) monokine activities from liver granuloma macrophages of Schistosoma mansoni-infected mice. J Immunol. 1987 Apr 15;138(8):2653–2662. [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Elford P. R., Stutzer A., Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990 Jul;5(7):781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Godleski J. J., Brain J. D. The origin of alveolar macrophages in mouse radiation chimeras. J Exp Med. 1972 Sep 1;136(3):630–643. doi: 10.1084/jem.136.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi A., Komuro T., Saizawa T., Sakamoto T., Watari E., Yokomuro K. The liver and the hematolymphoid system: I. The regulation of nylon-passed spleen cell proliferation by active factors released from syngeneic nonparenchymal liver cells. J Leukoc Biol. 1991 Oct;50(4):402–411. doi: 10.1002/jlb.50.4.402. [DOI] [PubMed] [Google Scholar]
- Malorny U., Michels E., Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243(2):421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Naito M., Takahashi K. The role of Kupffer cells in glucan-induced granuloma formation in the liver of mice depleted of blood monocytes by administration of strontium-89. Lab Invest. 1991 May;64(5):664–674. [PubMed] [Google Scholar]
- Patchen M. L., D'Alesandro M. M., Brook I., Blakely W. F., MacVittie T. J. Glucan: mechanisms involved in its "radioprotective" effect. J Leukoc Biol. 1987 Aug;42(2):95–105. doi: 10.1002/jlb.42.2.95. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Mabuchi A., Kuriya S., Sudo T., Aida T., Asano G., Shoji T., Yokomuro K. Production of granulocyte-macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). Reg Immunol. 1990;3(5):260–267. [PubMed] [Google Scholar]
- Sawyer R. T., Strausbauch P. H., Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982 Feb;46(2):165–170. [PubMed] [Google Scholar]
- Shikama Y., Kobayashi K., Kasahara K., Kaga S., Hashimoto M., Yoneya I., Hosoda S., Soejima K., Ide H., Takahashi T. Granuloma formation by artificial microparticles in vitro. Macrophages and monokines play a critical role in granuloma formation. Am J Pathol. 1989 Jun;134(6):1189–1199. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Morioka Y., Schultz L. D. Immunophenotypic and ultrastructural differentiation and maturation of nonlymphoid dendritic cells in osteopetrotic (op) mice with the total absence of macrophage colony stimulating factor activity. Adv Exp Med Biol. 1993;329:293–297. doi: 10.1007/978-1-4615-2930-9_49. [DOI] [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the kinetics of mononuclear phagocytes. J Exp Med. 1970 Mar 1;131(3):429–442. doi: 10.1084/jem.131.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Chang N. C., Strausbauch P. H., Morahan P. S. Differential effects of chronic monocyte depletion on macrophage populations. Lab Invest. 1983 Sep;49(3):291–298. [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Dougherty S., Evequoz V., Pluznik D. H., Wilder R. L., Hand A. R., Wahl L. M. T lymphocyte-dependent evolution of bacterial cell wall-induced hepatic granulomas. J Immunol. 1986 Oct 1;137(7):2199–2209. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Ansari A. A., Szperl M., Urbanowska E. Distinct in vivo functions of two macrophage subpopulations as evidenced by studies using macrophage-deficient op/op mouse. Eur J Immunol. 1992 Jul;22(7):1951–1954. doi: 10.1002/eji.1830220743. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Ratajczak M. Z., Ptasznik A., Sell K. W., Ahmed-Ansari A., Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992 Sep;20(8):1004–1010. [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- Yamada M., Naito M., Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol. 1990 Mar;47(3):195–205. [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]