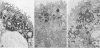Abstract
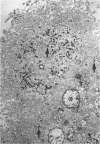
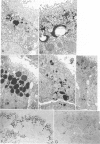
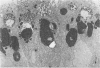
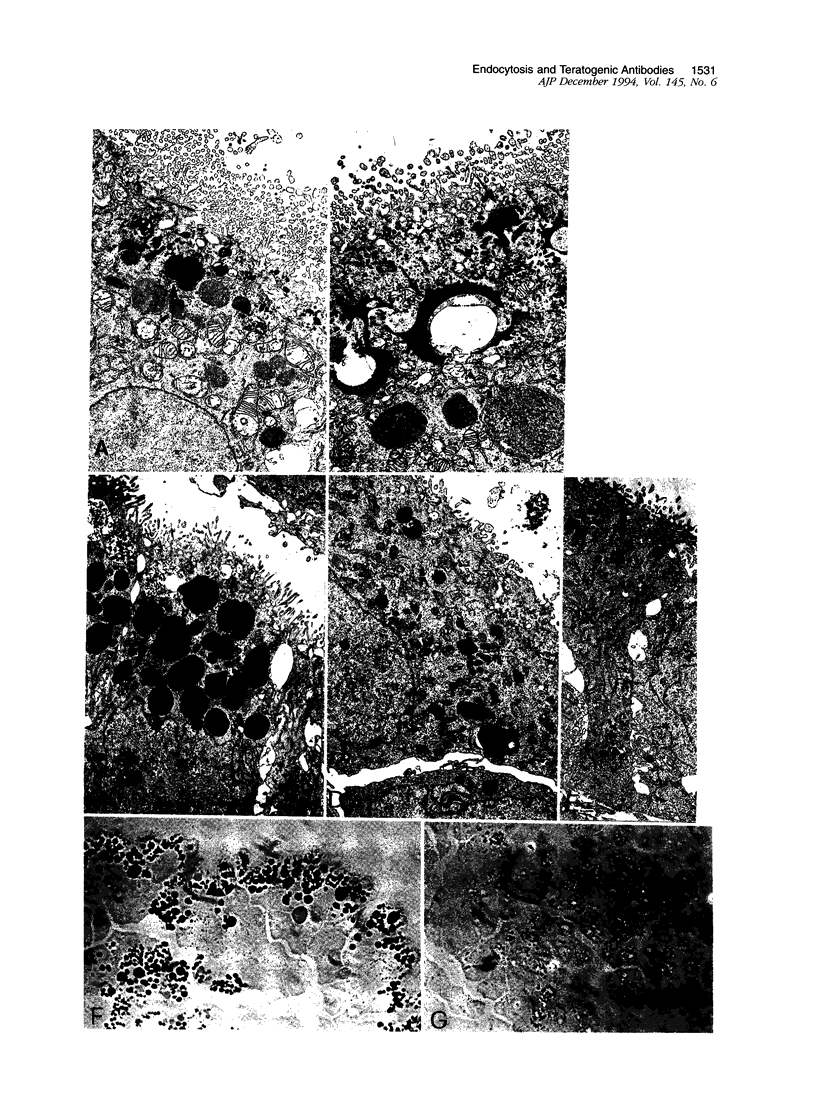
Previous studies have identified two high-molecular weight (280 and 330 kd) glycoproteins expressed by coated pits of the proximal renal tubule and yolk sac and have further established that, in vivo, antibodies to gp280 but not to gp330 induce fetal malformations. In the present study, we report the effect of these antibodies on the endocytic process by yolk sac visceral epithelial cells of rat embryos explanted at day 10 of gestation. Antibodies to gp280 markedly altered development of the yolk sac and embryo, induced malformations, inhibited by 40% the uptake of [14C] sucrose and perturbed the intracellular traffic of internalized proteins. Under control conditions, rat immunoglobulin G present in the culture medium was immunolocalized in lysosomes of epithelial cells, whereas in the presence of antibody, it was detected in small vesicles scattered through the apical cytoplasm. Alterations of the endocytic pathway were confirmed by experiments analyzing the uptake of peroxidase added to the medium for 2 to 60 minutes. The initial compartments of endocytosis visualized by peroxidase were increased in size and abnormal in shape and the transfer of the internalized peroxidase to the lysosomal compartment was delayed. In contrast, antibodies to gp330 had a minimal effect on embryonic development and did not induce fetal malformations. Endocytosis was only modestly altered; uptake of [14C] sucrose was decreased by 25%, and only minor modifications of the intracellular transit of peroxidase could be detected. We suggest that the key role of anti-gp280 antibodies is via trapping of the target antigen in the early endocytic compartment thus preventing its normal function in lysosomal transfer.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman D. A., Ornoy A., Jensen M., Arnon J., Brent R. L. Ultrastructure and function of the rat yolk sac: damage caused by teratogenic anti-VYS serum and recovery. Teratology. 1991 Aug;44(2):181–192. doi: 10.1002/tera.1420440206. [DOI] [PubMed] [Google Scholar]
- Birn H., Selhub J., Christensen E. I. Internalization and intracellular transport of folate-binding protein in rat kidney proximal tubule. Am J Physiol. 1993 Feb;264(2 Pt 1):C302–C310. doi: 10.1152/ajpcell.1993.264.2.C302. [DOI] [PubMed] [Google Scholar]
- Boutry J. M., Nivikoff A. B. Cytochemical studies on golgi apparatus, GERL, and lysosomes in neurons of dorsal root ganglia in mice. Proc Natl Acad Sci U S A. 1975 Feb;72(2):508–512. doi: 10.1073/pnas.72.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. A., Fabro S. Quantitation of rat embryonic development in vitro: a morphological scoring system. Teratology. 1981 Aug;24(1):65–78. doi: 10.1002/tera.1420240108. [DOI] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol. 1986 Mar;122(3):500–511. [PMC free article] [PubMed] [Google Scholar]
- Christensen E. I., Gliemann J., Moestrup S. K. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J Histochem Cytochem. 1992 Oct;40(10):1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- Christensen E. I. Rapid membrane recycling in renal proximal tubule cells. Eur J Cell Biol. 1982 Nov;29(1):43–49. [PubMed] [Google Scholar]
- Cui S. Y., Christensen E. I., Nielsen S. Membrane traffic after inhibition of endocytosis in renal proximal tubules. J Struct Biol. 1991 Dec;107(3):201–210. doi: 10.1016/1047-8477(91)90045-x. [DOI] [PubMed] [Google Scholar]
- Cui S., Christensen E. I. Three-dimensional organization of the vacuolar apparatus involved in endocytosis and membrane recycling of rat kidney proximal tubule cells. An electron-microscopic study of serial sections. Exp Nephrol. 1993 May-Jun;1(3):175–184. [PubMed] [Google Scholar]
- DAVID G., MERCIER-PAROT L., TUCHMANN-DUPLESSIS H. ACTION T'ERATOG'ENE D'H'ET'ERO-ANTICORPS TISSULAIRES. I. PRODUCTION DE MALFORMATIONS CHEZ LE RAT PAR ACTION D'UN S'ERUM ANTI-REIN) (FR) C R Seances Soc Biol Fil. 1963 Oct 5;157:939–942. [PubMed] [Google Scholar]
- Freeman S. J., Beck F., Lloyd J. B. The role of the visceral yolk sac in mediating protein utilization by rat embryos cultured in vitro. J Embryol Exp Morphol. 1981 Dec;66:223–234. [PubMed] [Google Scholar]
- Freeman S. J., Brent R. L., Lloyd J. B. The effect of teratogenic antiserum on yolk-sac function in rat embryos cultured in vitro. J Embryol Exp Morphol. 1982 Oct;71:63–74. [PubMed] [Google Scholar]
- Freeman S. J., Lloyd J. B. Evidence that protein ingested by the rat visceral yolk sac yields amino acids for synthesis of embryonic protein. J Embryol Exp Morphol. 1983 Feb;73:307–315. [PubMed] [Google Scholar]
- Freeman S. J., Lloyd J. B. Inhibition of proteolysis in rat yolk sac as a cause of teratogenesis. Effects of leupeptin in vitro and in vivo. J Embryol Exp Morphol. 1983 Dec;78:183–193. [PubMed] [Google Scholar]
- Gartung C., Braulke T., Hasilik A., von Figura K. Internalization of blocking antibodies against mannose-6-phosphate specific receptors. EMBO J. 1985 Jul;4(7):1725–1730. doi: 10.1002/j.1460-2075.1985.tb03842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatae T., Fujita M., Sagara H. Helical structure in the apical tubules of several absorbing epithelia. Kidney proximal tubule, visceral yolk sac and ductuli efferentes. Cell Tissue Res. 1986;244(1):39–46. doi: 10.1007/BF00218379. [DOI] [PubMed] [Google Scholar]
- Jensen M., Lloyd J. B., Koszalka T. R., Beckman D. A., Brent R. L. Preparation and developmental toxicity of monoclonal antibodies against rat visceral yolk sac antigens. Teratology. 1989 Nov;40(5):505–511. doi: 10.1002/tera.1420400513. [DOI] [PubMed] [Google Scholar]
- Jensen M., Lloyd J. B., Vega P., Koszalka T. R., Brent R. L. Multiple antigens in the rat visceral yolk sac induce teratogenic antisera. Teratology. 1991 Jun;43(6):601–608. doi: 10.1002/tera.1420430615. [DOI] [PubMed] [Google Scholar]
- Jollie W. P. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 1990 Apr;41(4):361–381. doi: 10.1002/tera.1420410403. [DOI] [PubMed] [Google Scholar]
- Jollie W. P. Ultrastructural studies of protein transfer across rodent yolk sac. Placenta. 1986 May-Jun;7(3):263–281. doi: 10.1016/s0143-4004(86)80164-3. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Hatae T. Three-dimensional representation and quantification of endosomes in the rat kidney proximal tubule cell. J Electron Microsc (Tokyo) 1991 Dec;40(6):411–415. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killisch I., Steinlein P., Römisch K., Hollinshead R., Beug H., Griffiths G. Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J Cell Sci. 1992 Sep;103(Pt 1):211–232. doi: 10.1242/jcs.103.1.211. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J., Livesey G., Williams K. E. Effects of microbial proteinase inhibitors on the degradation of endogenous and internalized proteins by rat yolk sacs. Biochem J. 1981 Apr 15;196(1):41–48. doi: 10.1042/bj1960041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung C. C., Cheewatrakoolpong B., Yan C. L. Teratogenic antibodies are directed against a coated-pit glycoprotein. Anat Rec. 1989 Apr;223(4):363–367. doi: 10.1002/ar.1092230403. [DOI] [PubMed] [Google Scholar]
- Leung C. C., DeSha D. L., Bui L., Cheewatrakoolpong B. Ultrastructural pathologic changes of rat extraembryonic visceral endodermal cells exposed to teratogenic antibodies in vivo. Histol Histopathol. 1988 Jan;3(1):49–55. [PubMed] [Google Scholar]
- Leung C. C. Isolation, partial characterization, and localization of a rat renal tubular glycoprotein antigen. Antibody-induced birth defects. J Exp Med. 1982 Aug 1;156(2):372–384. doi: 10.1084/jem.156.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. B. Cell physiology of the rat visceral yolk sac: a study of pinocytosis and lysosome function. Teratology. 1990 Apr;41(4):383–393. doi: 10.1002/tera.1420410404. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Nielsen S., Andreasen P., Jørgensen K. E., Nykjaer A., Røigaard H., Gliemann J., Christensen E. I. Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes. J Biol Chem. 1993 Aug 5;268(22):16564–16570. [PubMed] [Google Scholar]
- New D. A., Brent R. L. Effect of yolk-sac antibody on rat embryos grown in culture. J Embryol Exp Morphol. 1972 Jun;27(3):543–553. [PubMed] [Google Scholar]
- Nielsen S. Endocytosis in proximal tubule cells involves a two-phase membrane-recycling pathway. Am J Physiol. 1993 Apr;264(4 Pt 1):C823–C835. doi: 10.1152/ajpcell.1993.264.4.C823. [DOI] [PubMed] [Google Scholar]
- Nielsen S. Sorting and recycling efficiency of apical insulin binding sites during endocytosis in proximal tubule cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C810–C822. doi: 10.1152/ajpcell.1993.264.4.C810. [DOI] [PubMed] [Google Scholar]
- Nolan C. M., Creek K. E., Grubb J. H., Sly W. S. Antibody to the phosphomannosyl receptor inhibits recycling of receptor in fibroblasts. J Cell Biochem. 1987 Oct;35(2):137–151. doi: 10.1002/jcb.240350207. [DOI] [PubMed] [Google Scholar]
- Orlando R. A., Kerjaschki D., Kurihara H., Biemesderfer D., Farquhar M. G. gp330 associates with a 44-kDa protein in the rat kidney to form the Heymann nephritis antigenic complex. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6698–6702. doi: 10.1073/pnas.89.15.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Schrotz P., Bucci C., Gruenberg J. Plasticity of early endosomes. J Cell Sci. 1992 Oct;103(Pt 2):335–348. doi: 10.1242/jcs.103.2.335. [DOI] [PubMed] [Google Scholar]
- Ronco P., Melcion C., Geniteau M., Ronco E., Reininger L., Galceran M., Verroust P. Production and characterization of monoclonal antibodies against rat brush border antigens of the proximal convoluted tubule. Immunology. 1984 Sep;53(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Sahali D., Mulliez N., Chatelet F., Dupuis R., Ronco P., Verroust P. Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med. 1988 Jan 1;167(1):213–218. doi: 10.1084/jem.167.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahali D., Mulliez N., Chatelet F., Laurent-Winter C., Citadelle D., Sabourin J. C., Roux C., Ronco P., Verroust P. Comparative immunochemistry and ontogeny of two closely related coated pit proteins. The 280-kd target of teratogenic antibodies and the 330-kd target of nephritogenic antibodies. Am J Pathol. 1993 May;142(5):1654–1667. [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. I. Kinetics of uptake of (125I)polyvinylpyrrolidone by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):113–122. doi: 10.1083/jcb.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Roberts G., Kidston M. E., Beck F., Lloyd J. B. Inhibition of pinocytosis in rat yolk sac by trypan blue. Teratology. 1976 Dec;14(3):343–354. doi: 10.1002/tera.1420140310. [DOI] [PubMed] [Google Scholar]
- Willnow T. E., Goldstein J. L., Orth K., Brown M. S., Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem. 1992 Dec 25;267(36):26172–26180. [PubMed] [Google Scholar]