Abstract
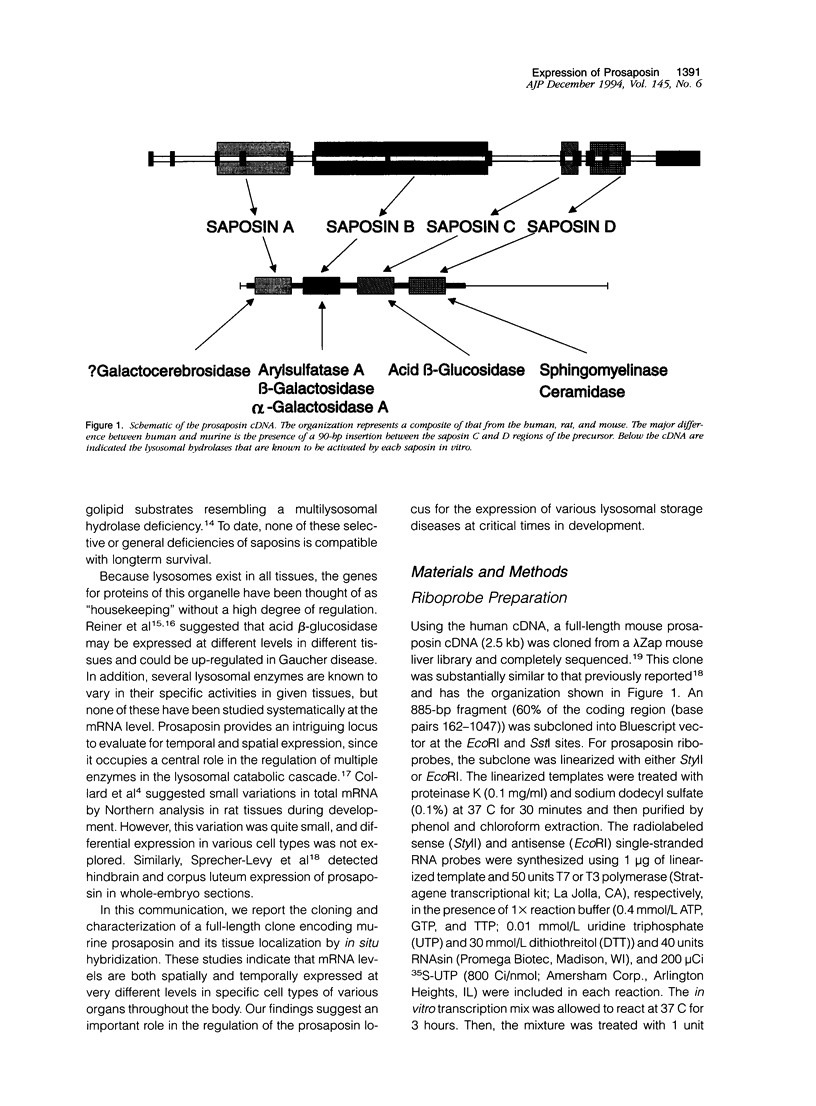
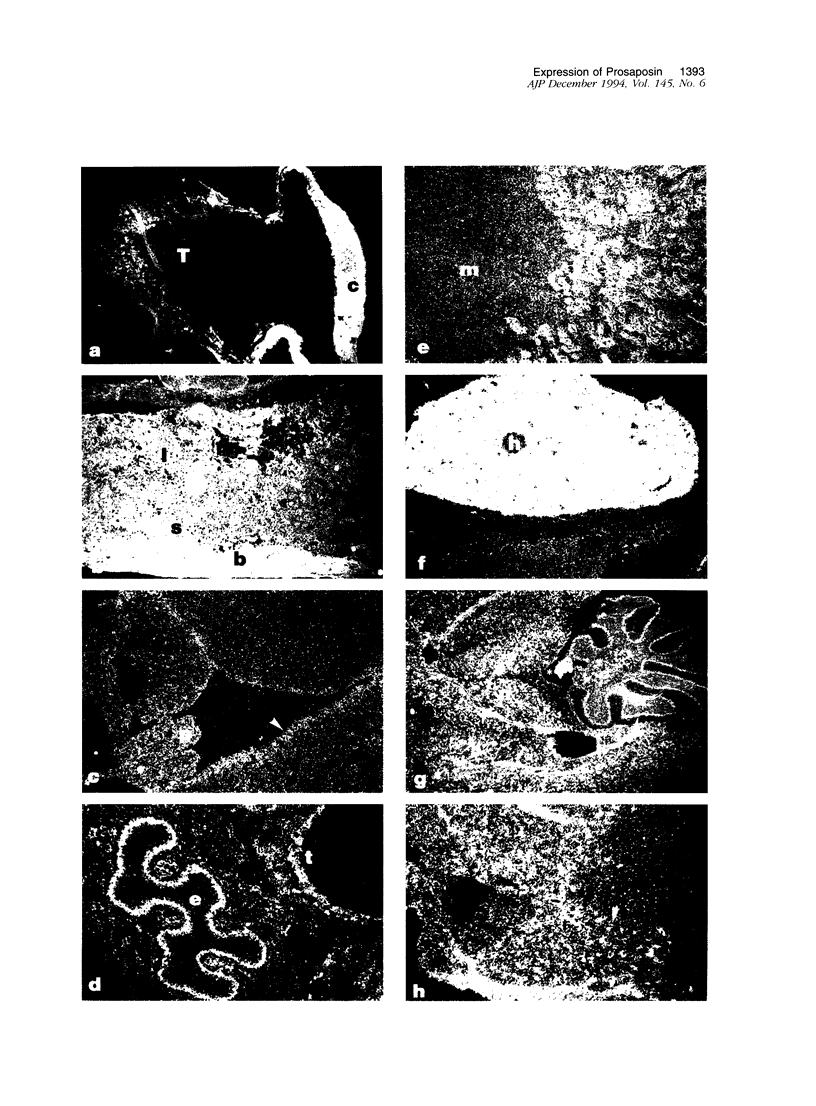
Prosaposin is a multifunctional locus in humans and mice that encodes in tandem and in the same reading frame four glycoprotein activators, or saposins, of lysosomal hydrolases. These ubiquitously expressed glycoproteins and the precursor, prosaposin, have been proposed to function in glycosphingolipid catabolic pathways and glycolipid transport. To characterize the temporal and spatial expression of the prosaposin locus, prenatal and postnatal mouse tissues were screened by in situ hybridization with a mouse antisense riboprobe for prosaposin. Prenatally, prosaposin mRNA was expressed differentially in the placenta and prominently in the decidua basalis and capsularis where expression was gestational age dependent. No other region of high-level expression was detectable in the prenatal mouse. In comparison, high-level differential expression of prosaposin was clearly evident postnatally in a variety of organs, including secretory epithelial cells of the choroid plexus, ependymal lining, upper trachea, esophagus, cortical tubules of the kidney, sertoli cells of the testes and epididymis. Discrete localization of prosaposin mRNA expression was also found in neurons of the cerebral cortex, cerebellar cortex, and lateral columns of the spinal cord as well as in hepatocytes of the mature liver. Very high levels of expression were found in specialized tissues including the Harderian glands and macrophages of lymph nodes, lungs, splenic tissue, and thymus. These studies indicate that the expression of the prosaposin locus, a presumed "housekeeping" gene, is under tissue- and cell-specific differential control. The spatial organization of expression suggests a role for this locus in the expression of glycosphingolipid-storage diseases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrides T., Moses A. C., Smith R. J. Developmental expression of receptors for insulin, insulin-like growth factor I (IGF-I), and IGF-II in rat skeletal muscle. Endocrinology. 1989 Feb;124(2):1064–1076. doi: 10.1210/endo-124-2-1064. [DOI] [PubMed] [Google Scholar]
- Aronow B. J., Lund S. D., Brown T. L., Harmony J. A., Witte D. P. Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):725–729. doi: 10.1073/pnas.90.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christomanou H., Chabás A., Pámpols T., Guardiola A. Activator protein deficient Gaucher's disease. A second patient with the newly identified lipid storage disorder. Klin Wochenschr. 1989 Oct 2;67(19):999–1003. doi: 10.1007/BF01716064. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Sylvester S. R., Tsuruta J. K., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat Sertoli cells: sequence similarity with the 70-kilodalton precursor to sulfatide/GM1 activator. Biochemistry. 1988 Jun 14;27(12):4557–4564. doi: 10.1021/bi00412a050. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Robertson E. J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991 Feb 22;64(4):849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Biosynthesis of the sulfatide/GM1 activator protein (SAP-1) in control and mutant cultured skin fibroblasts. Biochim Biophys Acta. 1986 Feb 28;875(3):554–562. doi: 10.1016/0005-2760(86)90077-9. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Synthesis and processing of sphingolipid activator protein-2 (SAP-2) in cultured human fibroblasts. J Biol Chem. 1986 Nov 15;261(32):15339–15343. [PubMed] [Google Scholar]
- Fürst W., Sandhoff K. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim Biophys Acta. 1992 Jun 5;1126(1):1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M., Soeda S., Kishimoto Y., O'Brien J. S. Binding and transport of gangliosides by prosaposin. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11254–11258. doi: 10.1073/pnas.89.23.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtschmidt H., Sandhoff K., Kwon H. Y., Harzer K., Nakano T., Suzuki K. Sulfatide activator protein. Alternative splicing that generates three mRNAs and a newly found mutation responsible for a clinical disease. J Biol Chem. 1991 Apr 25;266(12):7556–7560. [PubMed] [Google Scholar]
- Kishimoto Y., Hiraiwa M., O'Brien J. S. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res. 1992 Sep;33(9):1255–1267. [PubMed] [Google Scholar]
- Morimoto S., Yamamoto Y., O'Brien J. S., Kishimoto Y. Distribution of saposin proteins (sphingolipid activator proteins) in lysosomal storage and other diseases. Proc Natl Acad Sci U S A. 1990 May;87(9):3493–3497. doi: 10.1073/pnas.87.9.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Sandhoff K., Stümper J., Christomanou H., Suzuki K. Structure of full-length cDNA coding for sulfatide activator, a Co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J Biochem. 1989 Feb;105(2):152–154. doi: 10.1093/oxfordjournals.jbchem.a122629. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kretz K. A., Dewji N., Wenger D. A., Esch F., Fluharty A. L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988 Aug 26;241(4869):1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- Rafi M. A., de Gala G., Zhang X. L., Wenger D. A. Mutational analysis in a patient with a variant form of Gaucher disease caused by SAP-2 deficiency. Somat Cell Mol Genet. 1993 Jan;19(1):1–7. doi: 10.1007/BF01233949. [DOI] [PubMed] [Google Scholar]
- Reiner O., Dagan O., Horowitz M. Human sphingolipid activator protein-1 and sphingolipid activator protein-2 are encoded by the same gene. J Mol Neurosci. 1989;1(4):225–233. [PubMed] [Google Scholar]
- Reiner O., Horowitz M. Differential expression of the human glucocerebrosidase-coding gene. Gene. 1988 Dec 20;73(2):469–478. doi: 10.1016/0378-1119(88)90511-2. [DOI] [PubMed] [Google Scholar]
- Reiner O., Wigderson M., Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988 Mar;7(2):107–116. doi: 10.1089/dna.1988.7.107. [DOI] [PubMed] [Google Scholar]
- Rorman E. G., Grabowski G. A. Molecular cloning of a human co-beta-glucosidase cDNA: evidence that four sphingolipid hydrolase activator proteins are encoded by single genes in humans and rats. Genomics. 1989 Oct;5(3):486–492. doi: 10.1016/0888-7543(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schmid B., Paton B. C., Sandhoff K., Harzer K. Metabolism of GM1 ganglioside in cultured skin fibroblasts: anomalies in gangliosidoses, sialidoses, and sphingolipid activator protein (SAP, saposin) 1 and prosaposin deficient disorders. Hum Genet. 1992 Jul;89(5):513–518. doi: 10.1007/BF00219176. [DOI] [PubMed] [Google Scholar]
- Schnabel D., Schröder M., Fürst W., Klein A., Hurwitz R., Zenk T., Weber J., Harzer K., Paton B. C., Poulos A. Simultaneous deficiency of sphingolipid activator proteins 1 and 2 is caused by a mutation in the initiation codon of their common gene. J Biol Chem. 1992 Feb 15;267(5):3312–3315. [PubMed] [Google Scholar]
- Schnabel D., Schröder M., Sandhoff K. Mutation in the sphingolipid activator protein 2 in a patient with a variant of Gaucher disease. FEBS Lett. 1991 Jun 17;284(1):57–59. doi: 10.1016/0014-5793(91)80760-z. [DOI] [PubMed] [Google Scholar]
- Senior P. V., Byrne S., Brammar W. J., Beck F. Expression of the IGF-II/mannose-6-phosphate receptor mRNA and protein in the developing rat. Development. 1990 May;109(1):67–73. doi: 10.1242/dev.109.1.67. [DOI] [PubMed] [Google Scholar]
- Sklar M. M., Thomas C. L., Municchi G., Roberts C. T., Jr, LeRoith D., Kiess W., Nissley P. Developmental expression of rat insulin-like growth factor-II/mannose 6-phosphate receptor messenger ribonucleic acid. Endocrinology. 1992 Jun;130(6):3484–3491. doi: 10.1210/endo.130.6.1317785. [DOI] [PubMed] [Google Scholar]
- Sprecher-Levy H., Orr-Urtreger A., Lonai P., Horowitz M. Murine prosaposin: expression in the reproductive system of a gene implicated in human genetic diseases. Cell Mol Biol (Noisy-le-grand) 1993 May;39(3):287–299. [PubMed] [Google Scholar]
- Witte D. P., Welch T. R., Beischel L. S. Detection and cellular localization of human C4 gene expression in the renal tubular epithelial cells and other extrahepatic epithelial sources. Am J Pathol. 1991 Oct;139(4):717–724. [PMC free article] [PubMed] [Google Scholar]
- Zhang X. L., Rafi M. A., DeGala G., Wenger D. A. The mechanism for a 33-nucleotide insertion in mRNA causing sphingolipid activator protein (SAP-1)-deficient metachromatic leukodystrophy. Hum Genet. 1991 Jun;87(2):211–215. doi: 10.1007/BF00204185. [DOI] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, Topology, and regulation. J Biol Chem. 1993 Mar 15;268(8):5341–5344. [PubMed] [Google Scholar]