Abstract
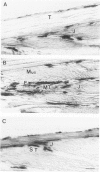
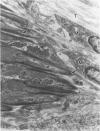
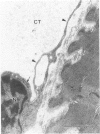
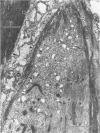
Modifications in muscle loading have been reported previously to result in increased numbers of mononucleated cells and changes in myofibril organization at myotendinous junctions (MTJs). The goals of this study were to determine the identity of those mononucleated cells and to examine the relationships between changes in their structure, location, and number with structural aspects of remodeling at MTJs experiencing modified loading. Soleus muscles from rats subjected to 10 days of hindlimb suspension were analyzed 0, 2, 4, and 7 days after return to weight bearing. Immunohistochemistry showed that ED1+, ED2+ and Ia+ macrophages were present at the MTJ and microtendon of control muscle. After reloading, ED2+ macrophages increased in number and size at MTJs and microtendons, indicating their activation. ED1+ cells showed no change in size or number whereas Ia+ cells were increased in size at day 7 of reloading. Electron microscopic observations showed that mononucleated cells near MTJs of control or suspended muscle were not highly active in protein synthesis or secretion. However, in reloaded muscle, mononucleated cells were found to be in close proximity to MTJs and to contain a high concentration of organelles associated with protein secretion. During these stages of reloading, extensive remodeling of myofibril-membrane associations occurred and nascent sarcomeres appeared in the MTJ regions of muscle fibers. Immunohistochemistry showed that during these stages of nascent sarcomere formation, there was renewed expression of developmental myosin heavy chain at MTJs, with this heavy chain appearing most prominently at the MTJ at day 7 of reloading. The activation and increased numbers of macrophages at MTJs and the close apposition of secretory cells to the MTJ membrane during remodeling lead us to propose that macrophage-derived factors may influence remodeling of MTJs in muscles experiencing modified loading.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almekinders L. C., Gilbert J. A. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med. 1986 Jul-Aug;14(4):303–308. doi: 10.1177/036354658601400411. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Witzemann V., Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990 Apr 5;344(6266):544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Fielding R. A., Fiatarone M. A., Orencole S. F., Dinarello C. A., Evans W. J. Increased interleukin 1 beta in human skeletal muscle after exercise. Am J Physiol. 1989 Aug;257(2 Pt 2):R451–R455. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- Clarke M. S., Khakee R., McNeil P. L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993 Sep;106(Pt 1):121–133. doi: 10.1242/jcs.106.1.121. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dix D. J., Eisenberg B. R. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. J Cell Biol. 1990 Nov;111(5 Pt 1):1885–1894. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J. V., McMenamin P. G., Holthouse I., Lumsden L., Liversidge J. Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye: implications for induction of autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):64–77. [PubMed] [Google Scholar]
- Frisén J., Risling M., Fried K. Distribution and axonal relations of macrophages in a neuroma. Neuroscience. 1993 Aug;55(4):1003–1013. doi: 10.1016/0306-4522(93)90314-6. [DOI] [PubMed] [Google Scholar]
- Goto M., Matsuno K., Yamaguchi Y., Ezaki T., Ogawa M. Proliferation kinetics of macrophage subpopulations in a rat experimental pancreatitis model. Arch Histol Cytol. 1993 Mar;56(1):75–82. doi: 10.1679/aohc.56.75. [DOI] [PubMed] [Google Scholar]
- Herman B., Pledger W. J. Platelet-derived growth factor-induced alterations in vinculin and actin distribution in BALB/c-3T3 cells. J Cell Biol. 1985 Apr;100(4):1031–1040. doi: 10.1083/jcb.100.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuff G., van der Ende M. B., Boutkan H., Prevoo W., Bayon L. G., Fleuren G. J., Beelen R. H., Meijer S., Dijkstra C. D. Macrophage populations in different stages of induced hepatic metastases in rats: an immunohistochemical analysis. Scand J Immunol. 1993 Jul;38(1):10–16. doi: 10.1111/j.1365-3083.1993.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Hines J. E., Johnson S. J., Burt A. D. In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol. 1993 Feb;142(2):511–518. [PMC free article] [PubMed] [Google Scholar]
- Honda H., Kimura H., Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990 Jun;70(2):272–277. [PMC free article] [PubMed] [Google Scholar]
- Honda H., Kimura H., Rostami A. Isolation and characterization of macrophages from rat embryonic muscle culture. J Leukoc Biol. 1992 Nov;52(5):537–544. doi: 10.1002/jlb.52.5.537. [DOI] [PubMed] [Google Scholar]
- Kida S., Steart P. V., Zhang E. T., Weller R. O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85(6):646–652. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- Krippendorf B. B., Riley D. A. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993 Jan;16(1):99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- Martinou J. C., Merlie J. P. Nerve-dependent modulation of acetylcholine receptor epsilon-subunit gene expression. J Neurosci. 1991 May;11(5):1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan I. S. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993 Apr;272(1):193–196. doi: 10.1007/BF00323586. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 1992 May;140(5):1097–1109. [PMC free article] [PubMed] [Google Scholar]
- Molony L., Armstrong L. Cytoskeletal reorganizations in human umbilical vein endothelial cells as a result of cytokine exposure. Exp Cell Res. 1991 Sep;196(1):40–48. doi: 10.1016/0014-4827(91)90454-3. [DOI] [PubMed] [Google Scholar]
- Monaco S., Gehrmann J., Raivich G., Kreutzberg G. W. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J Neurocytol. 1992 Sep;21(9):623–634. doi: 10.1007/BF01191724. [DOI] [PubMed] [Google Scholar]
- Nikolaou P. K., Macdonald B. L., Glisson R. R., Seaber A. V., Garrett W. E., Jr Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987 Jan-Feb;15(1):9–14. doi: 10.1177/036354658701500102. [DOI] [PubMed] [Google Scholar]
- Qwarnstrom E. E., Page R. C., Gillis S., Dower S. K. Binding, internalization, and intracellular localization of interleukin-1 beta in human diploid fibroblasts. J Biol Chem. 1988 Jun 15;263(17):8261–8269. [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1232–1236. doi: 10.1073/pnas.88.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T. A., Maley M. A., Grounds M. D., Papadimitriou J. M. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993 Aug;207(2):321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- Russell M. E., Adams D. H., Wyner L. R., Yamashita Y., Halnon N. J., Karnovsky M. J. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre B. A., Tidball J. G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol (1985) 1994 Jul;77(1):290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Thomason D. B., Herrick R. E., Surdyka D., Baldwin K. M. Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol (1985) 1987 Jul;63(1):130–137. doi: 10.1152/jappl.1987.63.1.130. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989 Jul;257(1):77–84. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., O'Halloran T., Burridge K. Talin at myotendinous junctions. J Cell Biol. 1986 Oct;103(4):1465–1472. doi: 10.1083/jcb.103.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G., Quan D. M. Reduction in myotendinous junction surface area of rats subjected to 4-day spaceflight. J Appl Physiol (1985) 1992 Jul;73(1):59–64. doi: 10.1152/jappl.1992.73.1.59. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Salem G., Zernicke R. Site and mechanical conditions for failure of skeletal muscle in experimental strain injuries. J Appl Physiol (1985) 1993 Mar;74(3):1280–1286. doi: 10.1152/jappl.1993.74.3.1280. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Spencer M. J. PDGF stimulation induces phosphorylation of talin and cytoskeletal reorganization in skeletal muscle. J Cell Biol. 1993 Nov;123(3):627–635. doi: 10.1083/jcb.123.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlin W. F., Kiely J. M., Gimbrone M. A., Jr Interleukin-8 induces changes in human neutrophil actin conformation and distribution: relationship to inhibition of adhesion to cytokine-activated endothelium. J Leukoc Biol. 1992 Jul;52(1):43–51. doi: 10.1002/jlb.52.1.43. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971 Nov;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Zamora A. J., Marini J. F. Tendon and myo-tendinous junction in an overloaded skeletal muscle of the rat. Anat Embryol (Berl) 1988;179(1):89–96. doi: 10.1007/BF00305103. [DOI] [PubMed] [Google Scholar]