Abstract
Although rat liver epithelial cell (RLEC) lines have been developed by a number of laboratories, the identity of the clonogenic nonparenchymal progenitors is unknown. To provide insight into the derivation of RLEC, we immunoisolated serosal liver mesothelial cells (LMC) and bile duct epithelial cells and attempted to propagate each epithelial cell population using culture conditions routinely employed to establish RLEC lines. Briefly, the selective reactivity of LMC with two bile duct cell surface markers, OC.2 and BD.2, was exploited to develop an immunocytochemical technique to isolate LMC. Livers were collagenase dissociated, the mesothelial capsule was "peeled" and digested with pronase to destroy contaminating hepatocytes, and rare biliary ductal epithelial cells were immunodepleted using OC.2. LMC were subsequently isolated by selective binding to magnetic beads adsorbed with BD.2 and cultured in supplemented Waymouths 752/1 media containing 10% fetal calf serum. Proliferating BD.2+ LMC rapidly formed epithelial-like monolayers that could be continuously subcultured after trypsinization. In contrast, attempts to establish cell lines from purified OC.2+ bile duct epithelial cells were unsuccessful. Results from reverse transcriptase polymerase chain reaction analysis confirmed that LMC expressed Wilms' tumor transcripts, a lineage marker for mesodermally-derived cells. In summary, our findings clearly demonstrate that LMC can be continuously propagated using culture conditions routinely employed to establish RLEC lines, an observation that supports the contention that some RLEC lines may be derived from LMC.
Full text
PDF




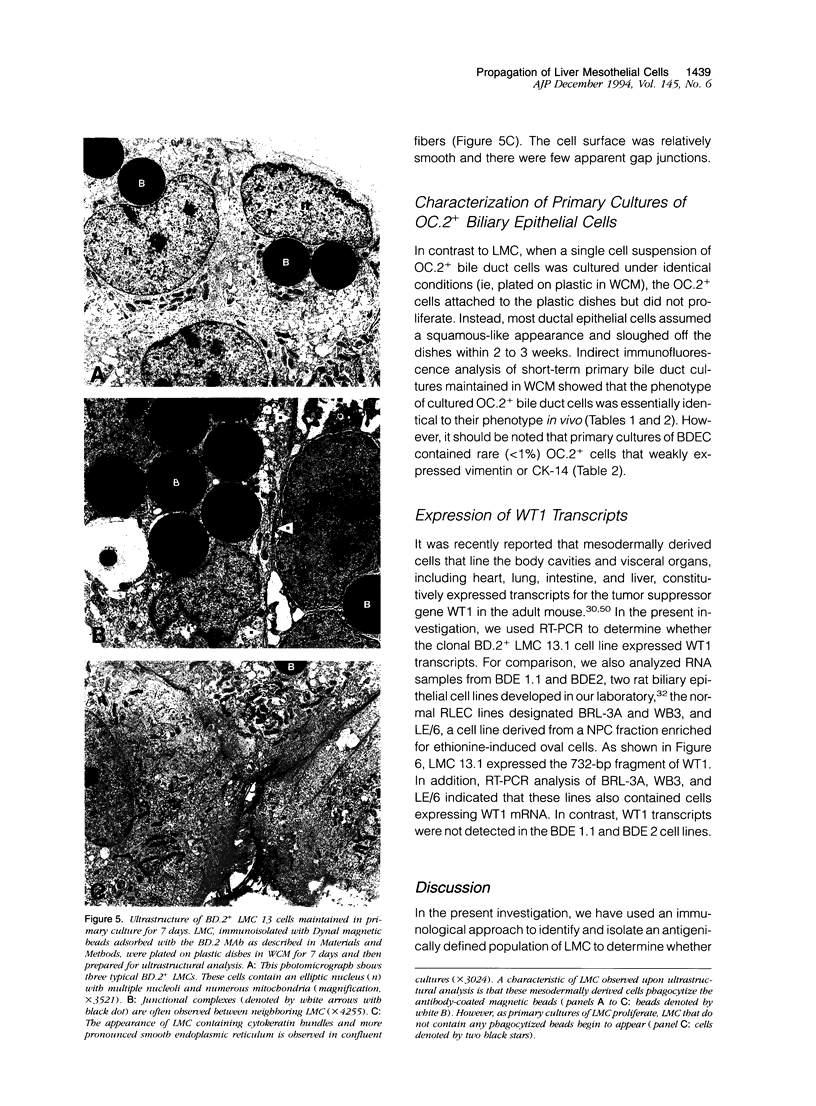




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Aragona E., Dabeva M., Salvi R., Shafritz D. A., Tavoloni N. Distribution of albumin and alpha-fetoprotein mRNAs in normal, hyperplastic, and preneoplastic rat liver. Am J Pathol. 1992 Sep;141(3):623–632. [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H. C., Parmelee D. C., Dunsford H. A., Sechi S., Thorgeirsson S. S. Keratin 14 protein in cultured nonparenchymal rat hepatic epithelial cells: characterization of keratin 14 and keratin 19 as antigens for the commonly used mouse monoclonal antibody OV-6. Mol Carcinog. 1993;7(1):60–66. doi: 10.1002/mc.2940070110. [DOI] [PubMed] [Google Scholar]
- Bisgaard H. C., Thorgeirsson S. S. Evidence for a common cell of origin for primitive epithelial cells isolated from rat liver and pancreas. J Cell Physiol. 1991 May;147(2):333–343. doi: 10.1002/jcp.1041470220. [DOI] [PubMed] [Google Scholar]
- Blouin R., Blouin M. J., Royal I., Grenier A., Roop D. R., Loranger A., Marceau N. Cytokeratin 14 expression in rat liver cells in culture and localization in vivo. Differentiation. 1992 Dec;52(1):45–54. doi: 10.1111/j.1432-0436.1992.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Borek C., Grob M., Burger M. M. Surface alterations in transformed epithelial and fibroblastic cells in culture: a disturbance of membrane degradation versus biosynthesis? Exp Cell Res. 1973 Mar 15;77(1):207–215. doi: 10.1016/0014-4827(73)90569-7. [DOI] [PubMed] [Google Scholar]
- Borek C. Neoplastic transformation in vitro of a clone of adult liver epithelial cells into differentiated hepatoma-like cells under conditions of nutritional stress. Proc Natl Acad Sci U S A. 1972 Apr;69(4):956–959. doi: 10.1073/pnas.69.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Goyette M., Yaswen P., Thompson N. L., Fausto N. Growth in culture and tumorigenicity after transfection with the ras oncogene of liver epithelial cells from carcinogen-treated rats. Cancer Res. 1987 Aug 1;47(15):4116–4124. [PubMed] [Google Scholar]
- Buckler A. J., Pelletier J., Haber D. A., Glaser T., Housman D. E. Isolation, characterization, and expression of the murine Wilms' tumor gene (WT1) during kidney development. Mol Cell Biol. 1991 Mar;11(3):1707–1712. doi: 10.1128/mcb.11.3.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessebeuf M., Olsson A., Bournot P., Desgres J., Guiguet M., Maume G., Maume B. F., Perissel B., Padieu P. Long term cell culture of rat liver epithelial cells retaining some hepatic functions. Biochimie. 1974;56(10):1365–1379. doi: 10.1016/s0300-9084(75)80023-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Akley N. J., Gould L. B., Libbus B. L. Characteristics of tumors and tumor cells cultured from experimental asbestos-induced mesotheliomas in rats. Am J Pathol. 1987 Dec;129(3):448–462. [PMC free article] [PubMed] [Google Scholar]
- Diamond L., McFall R., Tashiro Y., Sabatini D. The WIRL-3 rat liver cell lines and their transformed derivatives. Cancer Res. 1973 Nov;33(11):2627–2636. [PubMed] [Google Scholar]
- Dunsford H. A., Sell S. Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res. 1989 Sep 1;49(17):4887–4893. [PubMed] [Google Scholar]
- Evarts R. P., Hu Z., Fujio K., Marsden E. R., Thorgeirsson S. S. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993 Jul;4(7):555–561. [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hsia C. C., Dunsford H. A., Thorgeirsson S. S. Cellular and molecular changes in the early stages of chemical hepatocarcinogenesis in the rat. Cancer Res. 1990 Jun 1;50(11):3439–3444. [PubMed] [Google Scholar]
- Furukawa K., Shimada T., England P., Mochizuki Y., Williams G. M. Enrichment and characterization of clonogenic epithelial cells from adult rat liver and initiation of epithelial cell strains. In Vitro Cell Dev Biol. 1987 May;23(5):339–348. doi: 10.1007/BF02620990. [DOI] [PubMed] [Google Scholar]
- Garfield S., Huber B. E., Nagy P., Cordingley M. G., Thorgeirsson S. S. Neoplastic transformation and lineage switching of rat liver epithelial cells by retrovirus-associated oncogenes. Mol Carcinog. 1988;1(3):189–195. doi: 10.1002/mc.2940010307. [DOI] [PubMed] [Google Scholar]
- Germain L., Goyette R., Marceau N. Differential cytokeratin and alpha-fetoprotein expression in morphologically distinct epithelial cells emerging at the early stage of rat hepatocarcinogenesis. Cancer Res. 1985 Feb;45(2):673–681. [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Green K. J., Parry D. A., Steinert P. M., Virata M. L., Wagner R. M., Angst B. D., Nilles L. A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem. 1990 Feb 15;265(5):2603–2612. [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem. 1983;53-54(1-2):23–33. doi: 10.1007/BF00225244. [DOI] [PubMed] [Google Scholar]
- Grisham J. W., Coleman W. B., Smith G. J. Isolation, culture, and transplantation of rat hepatocytic precursor (stem-like) cells. Proc Soc Exp Biol Med. 1993 Dec;204(3):270–279. doi: 10.3181/00379727-204-43663. [DOI] [PubMed] [Google Scholar]
- Herrinig A. S., Raychaudhuri R., Kelley S. P., Iype P. T. Repeated establishment of diploid epithelial cell cultures from normal and partially hepatectomized rats. In Vitro. 1983 Jul;19(7):576–588. doi: 10.1007/BF02619606. [DOI] [PubMed] [Google Scholar]
- Hixson D. C., Allison J. P. Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res. 1985 Aug;45(8):3750–3760. [PubMed] [Google Scholar]
- Hixson D. C., Faris R. A., Thompson N. L. An antigenic portrait of the liver during carcinogenesis. Pathobiology. 1990;58(2):65–77. doi: 10.1159/000163565. [DOI] [PubMed] [Google Scholar]
- Hu Z., Evarts R. P., Fujio K., Marsden E. R., Thorgeirsson S. S. Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol. 1993 Jun;142(6):1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Iype P. T., Baldwin R. W., Glaves D. Cultures from adult rat liver cells. II. Demonstration of organ-specific cell surface antigens on cultured cells from normal liver. Br J Cancer. 1972 Feb;26(1):6–9. doi: 10.1038/bjc.1972.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iype P. T. Cultures from adult rat liver cells. I. Establishment of monolayer cell-cultures from normal liver. J Cell Physiol. 1971 Oct;78(2):281–288. doi: 10.1002/jcp.1040780217. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Lane R. D., Crissman R. S., Ginn S. High efficiency fusion procedure for producing monoclonal antibodies against weak immunogens. Methods Enzymol. 1986;121:183–192. doi: 10.1016/0076-6879(86)21017-4. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Fausto N. Multiple alpha-fetoprotein RNAs in adult rat liver: cell type-specific expression and differential regulation. Cancer Res. 1991 Sep 1;51(17):4656–4664. [PubMed] [Google Scholar]
- Marceau N., Germain L., Goyette R., Noël M., Gourdeau H. Cell of origin of distinct cultured rat liver epithelial cells, as typed by cytokeratin and surface component selective expression. Biochem Cell Biol. 1986 Aug;64(8):788–802. doi: 10.1139/o86-107. [DOI] [PubMed] [Google Scholar]
- Miyazaki M., Wahid S., Miyano K., Yabe T., Sato J. Expression of gamma-glutamyltranspeptidase in cultures of spontaneously and chemically transformed rat liver cells. Int J Cancer. 1983 Sep 15;32(3):373–377. doi: 10.1002/ijc.2910320318. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Drevon C., Tomatis L. Production of epithelial and mesenchymal tumours with rat liver cells transformed in vitro. Int J Cancer. 1975 Oct 15;16(4):550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- Nissley S. P., Short P. A., Rechler M. M., Podskalny J. M., Coon H. G. Proliferation of Buffalo rat liver cells in serum-free medium does not depend upon multiplication-stimulating activity (MSA). Cell. 1977 Jun;11(2):441–446. doi: 10.1016/0092-8674(77)90062-9. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Gerschenson L. E., DiPaolo J. A. Carcinomas from rat liver cells transformed spontaneously in culture. Cancer Res. 1972 Apr;32(4):877–879. [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sharma P. M., Yang X., Bowman M., Roberts V., Sukumar S. Molecular cloning of rat Wilms' tumor complementary DNA and a study of messenger RNA expression in the urogenital system and the brain. Cancer Res. 1992 Nov 15;52(22):6407–6412. [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Suzuki Y. Pathology of human malignant mesothelioma. Semin Oncol. 1981 Sep;8(3):268–282. [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W. Hepatocarcinomas, cholangiocarcinomas, and hepatoblastomas produced by chemically transformed cultured rat liver epithelial cells. A light- and electron-microscopic analysis. Am J Pathol. 1987 Apr;127(1):168–181. [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Smith J. D., Nelson K. G., Grisham J. W. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984 Sep;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- Whitaker D., Papadimitriou J. M., Walters M. N. The mesothelium; techniques for investigating the origin, nature and behaviour of mesothelial cells. J Pathol. 1980 Nov;132(3):263–271. doi: 10.1002/path.1711320308. [DOI] [PubMed] [Google Scholar]
- Williams A. O., Huggett A. C., Thorgeirsson S. S. Pathology of spontaneous and oncogene transformed rat liver epithelial cells and derived tumours in nude mice. Int J Exp Pathol. 1992 Feb;73(1):99–114. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Gunn J. M. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974 Nov;89(1):139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Weisburger E. K., Weisburger J. H. Isolation and long-term cell culture of epithelial-like cells from rat liver. Exp Cell Res. 1971 Nov;69(1):106–112. doi: 10.1016/0014-4827(71)90316-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Faris R. A., Hixson D. C. Long-term culture and characteristics of normal rat liver bile duct epithelial cells. Gastroenterology. 1993 Mar;104(3):840–852. doi: 10.1016/0016-5085(93)91021-9. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]