Abstract
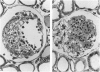
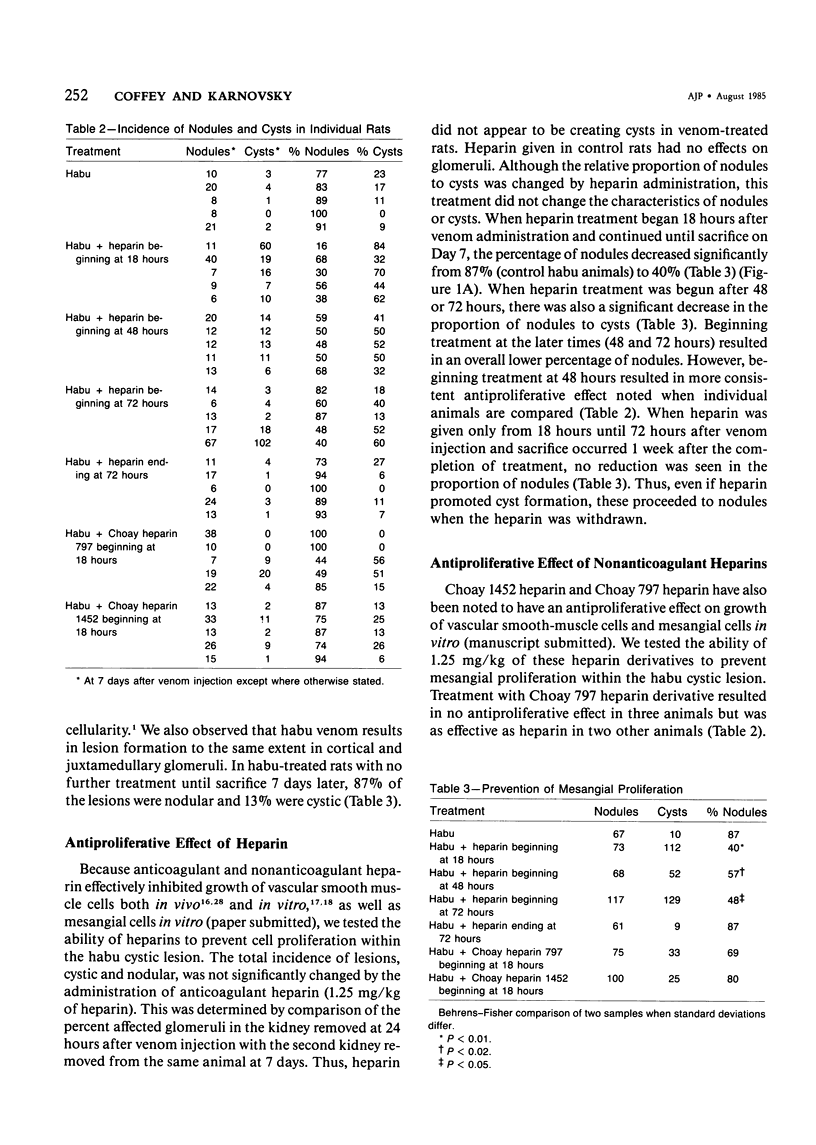
The authors have investigated the ability of anticoagulant heparin and nonanticoagulant heparin to inhibit mesangial-cell proliferation after the administration of habu (Trimeresurus flavorivids) snake venom to rats. Rats given injected habu venom exhibited glomerular capillary cystic lesions 6 to 24 hours later, and marked mesangial proliferation was noted within the cyst after 3 days. At 7 days 87% of these lesions (nodules) contained primarily mesangial cells embedded in a dense matrix and fibrin. A decrease in the frequency of nodules and the persistence of cysts indicate effective antiproliferative treatment. When anticoagulant heparin treatment extended from 18 hours after venom administration until sacrifice at 7 days, the percentage of nodules was reduced to 40%. Nonanticoagulant heparins resulted in some, but inconsistent, inhibition of mesangial-cell proliferation. The mechanism of the antiproliferative action of heparin on mesangial cells is not known but may be similar to that for vascular smooth muscle growth regulation. The authors suggest that endogenous heparin in the glomerular basement membrane and mesangial matrix may exert an antiproliferative effect under normal conditions. Loss of this inhibition due to glomerular damage might be reversed by the addition of exogenous heparin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAJAS L., LATTA H. A three-dimensional study of the juxtaglomerular apparatus in the rat. Light and electron microscopic observations. Lab Invest. 1963 Mar;12:257–269. [PubMed] [Google Scholar]
- Barajas L. The ultrastructure of the juxtaglomerular apparatus as disclosed by three-dimensional reconstructions from serial sections. The anatomical relationship between the tubular and vascular components. J Ultrastruct Res. 1970 Oct;33(1):116–147. doi: 10.1016/s0022-5320(70)90121-8. [DOI] [PubMed] [Google Scholar]
- Barry D. N., Bowness J. M. Identification and turnover of glycosaminoglycans in rat kidneys. Can J Biochem. 1975 Jun;53(6):713–720. doi: 10.1139/o75-098. [DOI] [PubMed] [Google Scholar]
- Boulton Jones J. M., Tulloch I., Dore B., McLay A. Changes in the glomerular capillary wall induced by lymphocyte products and serum of nephrotic patients. Clin Nephrol. 1983 Aug;20(2):72–77. [PubMed] [Google Scholar]
- Bradfield J. W., Cattell V., Smith J. The mesangial cell in glomerulonephritis. II. Mesangial proliferation caused by Habu snake venom in the rat. Lab Invest. 1977 May;36(5):487–492. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell V., Bradfield J. W. Focal mesangial proliferative glomerulonephritis in the rat caused by habu snake venom. A morphologic study. Am J Pathol. 1977 Jun;87(3):511–524. [PMC free article] [PubMed] [Google Scholar]
- Cattell V. Focal mesangial proliferative glomerulonephritis in the rat caused by Habu snake venom: the role of platelets. Br J Exp Pathol. 1979 Apr;60(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Cattell V., Mehotra A. Focal mesangial proliferative glomerulonephritis in the rat caused by Habu venom: the effect of antiplatelet agents. Br J Exp Pathol. 1980 Jun;61(3):310–314. [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Karnovsky M. J. Failure of certain antiplatelet drugs to affect myointimal thickening following arterial endothelial injury in the rat. Lab Invest. 1977 Apr;36(4):452–464. [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Foidart J. B., Goffinet G., Dubois C., Dechenne C., Mahieu P. R. Isolation and characterization of rat renal glomerular cells in vitro. Ren Physiol. 1980;3(1-6):174–182. doi: 10.1159/000172759. [DOI] [PubMed] [Google Scholar]
- Foidart J. B., Pirard Y. S., Winand R. J., Mahieu P. R. Tissue culture of normal rat glomeruli: glycosaminoglycan biosynthesis by homogeneous epithelial and mesangial cell populations. Ren Physiol. 1980;3(1-6):169–173. [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Johnston W. H., Latta H. Acute focal glomerulonephritis in the rabbit induced by injection of Saccharomyces cerevisiae. An electron microscopic study. Lab Invest. 1972 Jun;26(6):741–754. [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L., Rosenzweig L. J. Distribution of sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Eur J Cell Biol. 1983 Sep;31(2):290–295. [PubMed] [Google Scholar]
- Kondo Y., Akikusa B. Chronic Masugi nephritis in the rat. An electron microscopic study on evolution and consequences of glomerular capsular adhesions. Acta Pathol Jpn. 1982 Mar;32(2):231–242. [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Okabayashi A. Cellular aspects of rabbit Masugi nephritis. III. Mesangial changes. Lab Invest. 1976 Apr;34(4):363–371. [PubMed] [Google Scholar]
- Kreisberg J. I., Hoover R. L., Karnovsky M. J. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978 Jul;14(1):21–30. doi: 10.1038/ki.1978.86. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Wayne D. B., Karnovsky M. J. Rapid and focal loss of negative charge associated with mononuclear cell infiltration early in nephrotoxic serum nephritis. Kidney Int. 1979 Sep;16(3):290–300. doi: 10.1038/ki.1979.131. [DOI] [PubMed] [Google Scholar]
- Kurozumi T., Tanaka K., Kido M., Shoyama Y. Monocrotaline-induced renal lesions. Exp Mol Pathol. 1983 Dec;39(3):377–386. doi: 10.1016/0014-4800(83)90066-7. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. The juxtaglomerular apparatus as studied electron microscopically. J Ultrastruct Res. 1962 Jun;6:547–561. doi: 10.1016/s0022-5320(62)80009-4. [DOI] [PubMed] [Google Scholar]
- Lawler W. Experimental mesangial proliferative glomerulopathy. J Pathol. 1981 Feb;133(2):107–122. doi: 10.1002/path.1711330203. [DOI] [PubMed] [Google Scholar]
- McCully K. S., Rinehimer L. A., Gillies C. G., Hopfer S. M., Sunderman F. W., Jr Erythrocytosis, glomerulomegaly, mesangial hyperplasia, sialyl hyperplasia, and arteriosclerosis induced in rats by nickel subsulfide. Virchows Arch A Pathol Anat Histol. 1982;394(3):207–220. doi: 10.1007/BF00430666. [DOI] [PubMed] [Google Scholar]
- McEnery P. T., Strife C. F. Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am. 1982 Aug;29(4):875–894. [PubMed] [Google Scholar]
- Melcion C., Lachman L., Killen P. D., Morel-Maroger L., Striker G. E. Mesangial cells, effect of monocyte products on proliferation and matrix synthesis. Transplant Proc. 1982 Sep;14(3):559–564. [PubMed] [Google Scholar]
- Murphy W. M., Jukkola A. F., Roy S., 3rd Nephrotic syndrome with mesangial-cell proliferation in children--a distinct entity? Am J Clin Pathol. 1979 Jul;72(1):42–47. doi: 10.1093/ajcp/72.1.42. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Hirose S., Hamashima Y. Proliferation of cultured rabbit renal glomerular cells stimulated by platelet factor. Acta Pathol Jpn. 1980 Jan;30(1):1–7. doi: 10.1111/j.1440-1827.1980.tb01300.x. [DOI] [PubMed] [Google Scholar]
- Nörgaard J. O. Cellular outgrowth from isolated glomeruli. Origin and characterization. Lab Invest. 1983 May;48(5):526–542. [PubMed] [Google Scholar]
- Olson J. L. Role of heparin as a protective agent following reduction of renal mass. Kidney Int. 1984 Feb;25(2):376–382. doi: 10.1038/ki.1984.27. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAGUCHI H., KAWAMURA S. ELECTRON MICROSCOPIC OBSERVATIONS OF THE MESANGIOLYSIS. THE TOXIC EFFECTS OF THE "HABU SNAKE" VENOM ON THE RENAL GLOMERULUS. Keio J Med. 1963 Jun;12:99–111. [PubMed] [Google Scholar]
- Uff J. S., Mitcheson H. D., Pussell B. A., Brill M., Castro J. E. Proliferative glomerulonephritis in mice given intravenous Corynebacterium parvum. J Pathol. 1981 Feb;133(2):89–105. doi: 10.1002/path.1711330202. [DOI] [PubMed] [Google Scholar]
- VASSALLI P., MCCLUSKEY R. T. THE PATHOGENIC ROLE OF THE COAGULATION PROCESS IN RABBIT MASUGI NEPHRITIS. Am J Pathol. 1964 Oct;45:653–677. [PMC free article] [PubMed] [Google Scholar]
- VASSALLI P., SIMON G., ROUILLER C. ELECTRON MICROSCOPIC STUDY OF GLOMERULAR LESIONS RESULTING FROM INTRAVASCULAR FIBRIN FORMATION. Am J Pathol. 1963 Oct;43:579–617. [PMC free article] [PubMed] [Google Scholar]
- Waldherr R., Gubler M. C., Levy M., Broyer M., Habib R. The significance of pure diffuse mesangial proliferation in idiopathic nephrotic syndrome. Clin Nephrol. 1978 Nov;10(5):171–179. [PubMed] [Google Scholar]
- Wilson S. K., Solez K., Boitnott J. K., Heptinstall R. H. The effects of heparin treatment on hypertension and vascular lesions in stroke-prone spontaneously hypertensive rats. Am J Pathol. 1981 Jan;102(1):62–71. [PMC free article] [PubMed] [Google Scholar]