Abstract
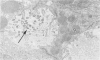
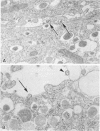
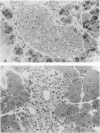
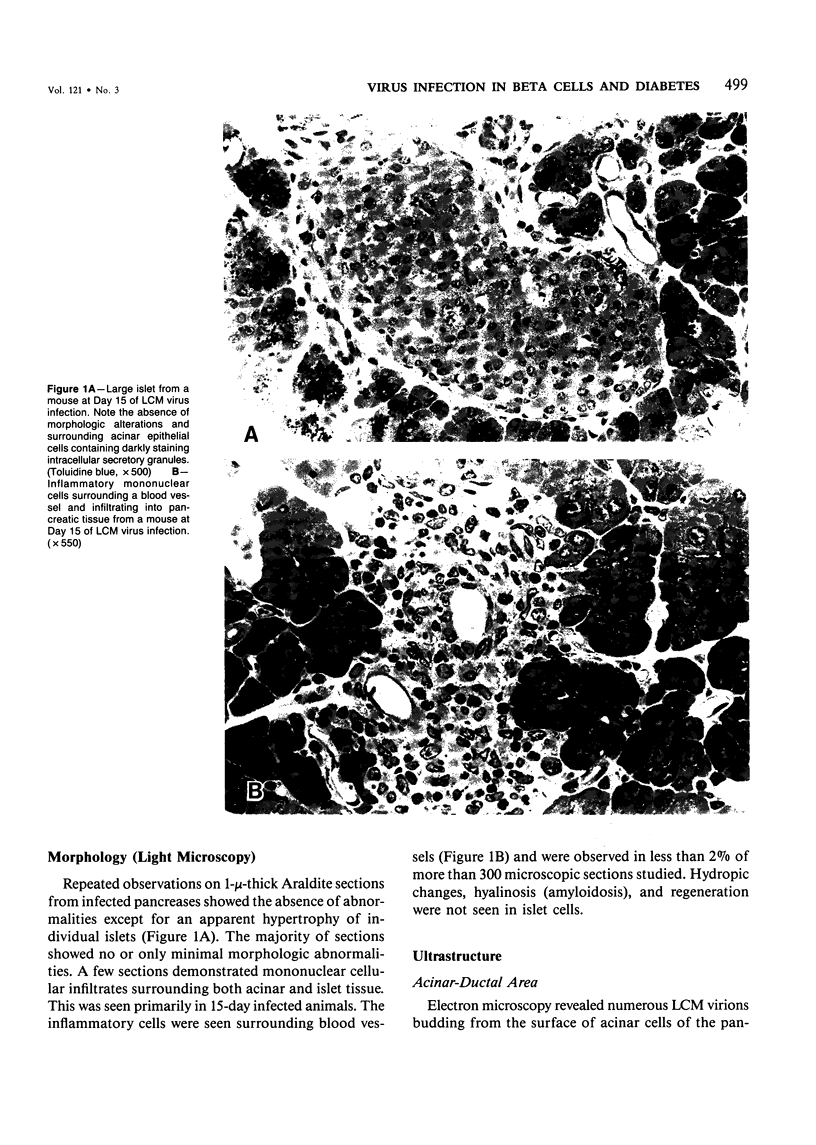
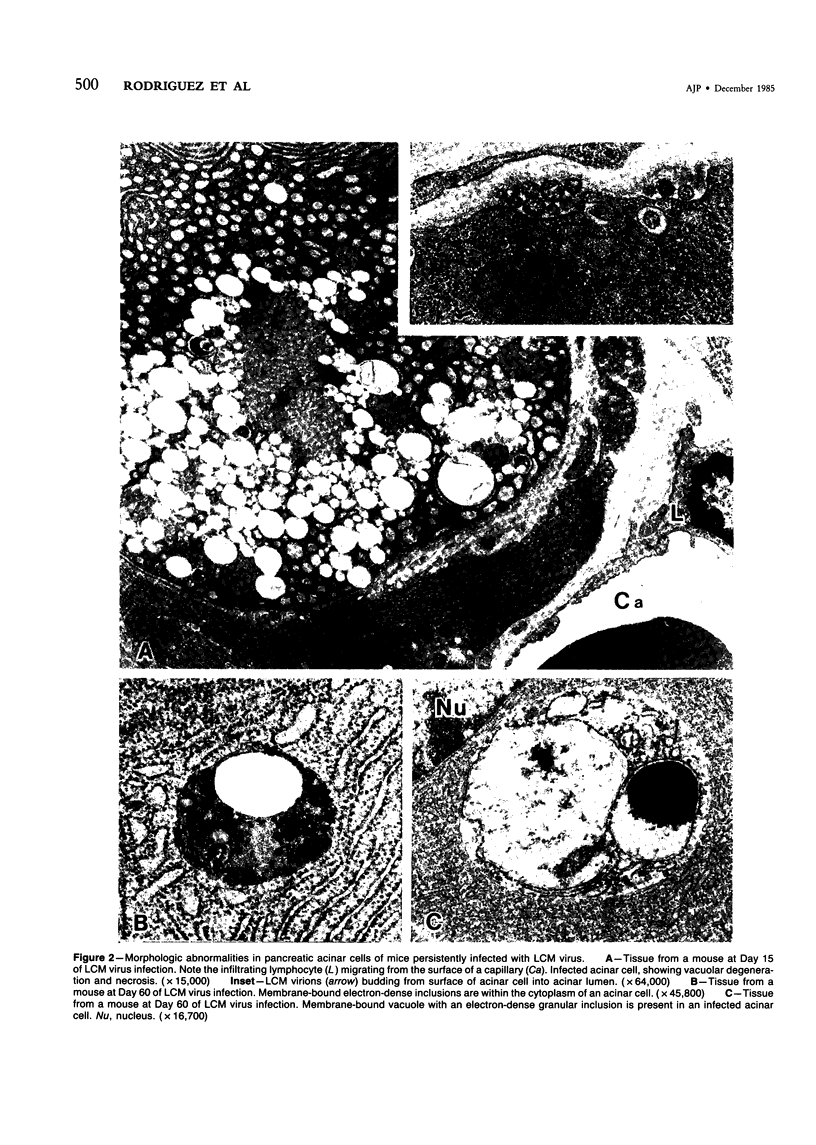
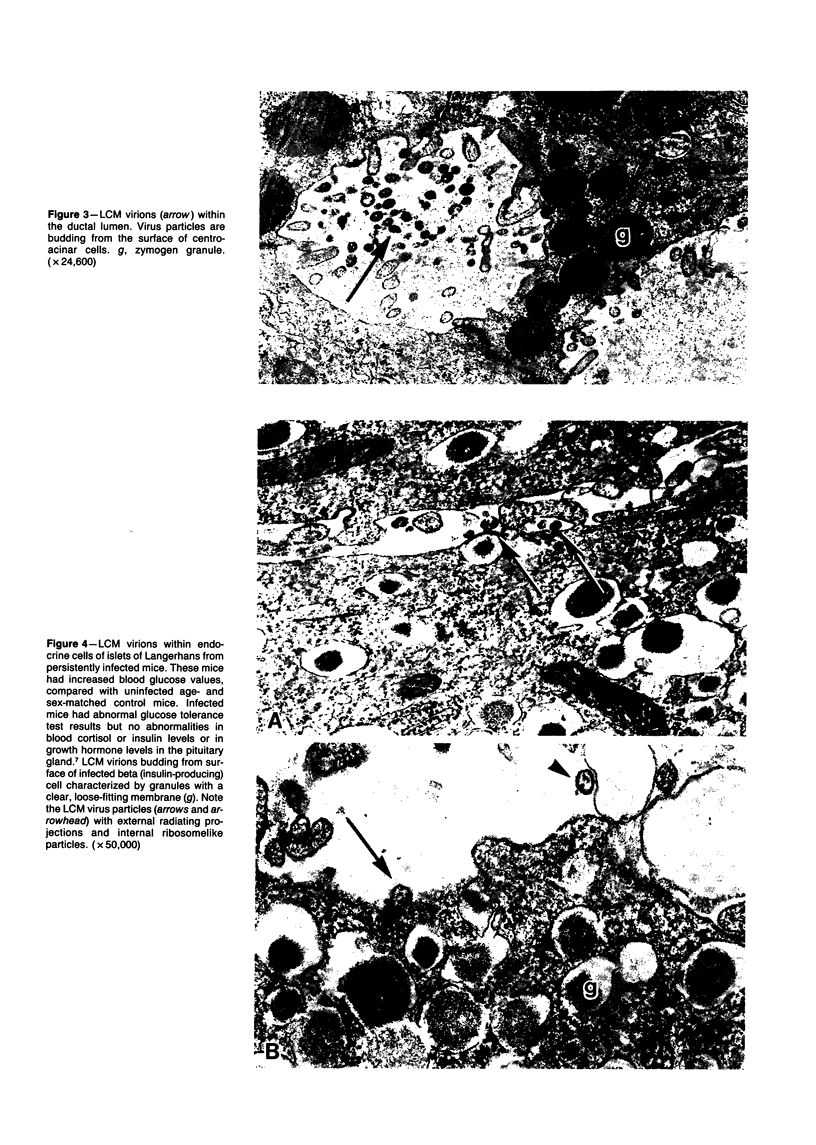
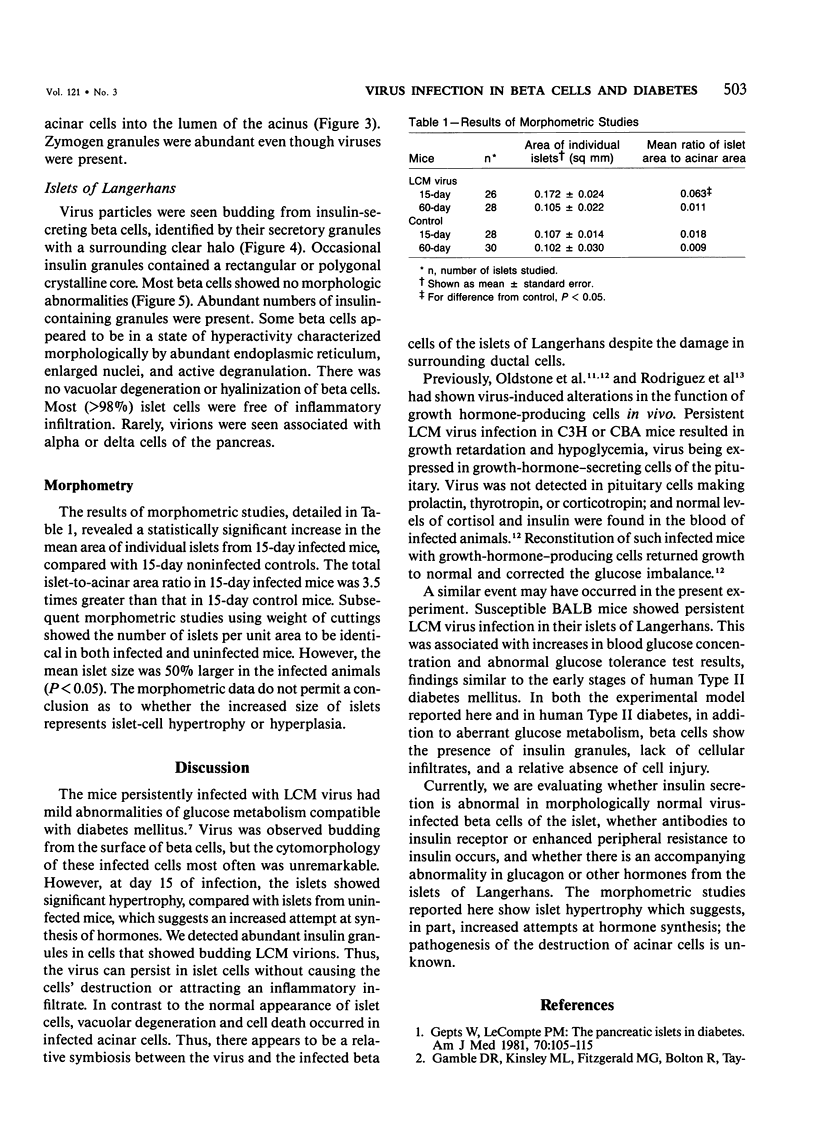
Persistence of lymphocytic choriomeningitis (LCM) virus in the islets of Langerhans was associated with mild hyperglycemia and abnormal glucose tolerance test results. Early histopathologic events consisted of occasional perivascular inflammatory mononuclear cells around both islet and acinar cells. Morphometric studies showed an increase in the size of islets from virus-infected mice. By electron microscopy, LCM virions were found within infected beta cells. Cytolytic injury of beta cells was minimal and did not account for the abnormalities of glucose metabolism. In contrast to the findings in islets, ultrastructural studies of acinar cells revealed LCM virions in abundance, vacuolar degeneration, and intracytoplasmic inclusions. This study extends the previous observation that LCM virus infection may persist in beta cells of the islets of Langerhans without causing structural injury but be associated with abnormalities resembling the chemical and histopathologic features of the early stage of Type II (adult-onset) human diabetes mellitus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Gamble D. R., Kinsley M. L., FitzGerald M. G., Bolton R., Taylor K. W. Viral antibodies in diabetes mellitus. Br Med J. 1969 Sep 13;3(5671):627–630. doi: 10.1136/bmj.3.5671.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble D. R., Taylor K. W., Cumming H. Coxsackie viruses and diabetes mellitus. Br Med J. 1973 Nov 3;4(5887):260–262. doi: 10.1136/bmj.4.5887.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble D. R., Taylor K. W. Seasonal incidence of diabetes mellitus. Br Med J. 1969 Sep 13;3(5671):631–633. doi: 10.1136/bmj.3.5671.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepts W., Lecompte P. M. The pancreatic islets in diabetes. Am J Med. 1981 Jan;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Rodriguez M., Daughaday W. H., Lampert P. W. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature. 1984 Jan 19;307(5948):278–281. doi: 10.1038/307278a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Sinha Y. N., Blount P., Tishon A., Rodriguez M., von Wedel R., Lampert P. W. Virus-induced alterations in homeostasis: alteration in differentiated functions of infected cells in vivo. Science. 1982 Dec 10;218(4577):1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Southern P., Rodriquez M., Lampert P. Virus persists in beta cells of islets of Langerhans and is associated with chemical manifestations of diabetes. Science. 1984 Jun 29;224(4656):1440–1443. doi: 10.1126/science.6203172. [DOI] [PubMed] [Google Scholar]
- Onodera T., Ray U. R., Melez K. A., Suzuki H., Toniolo A., Notkins A. L. Virus-induced diabetes mellitus: autoimmunity and polyendocrine disease prevented by immunosuppression. Nature. 1982 May 6;297(5861):66–68. doi: 10.1038/297066a0. [DOI] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., von Wedel R. J., Garrett R. S., Lampert P. W., Oldstone M. B. Pituitary dwarfism in mice persistently infected with lymphocytic choriomeningitis virus. Lab Invest. 1983 Jul;49(1):48–53. [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]