Abstract
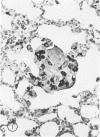
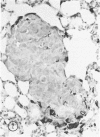
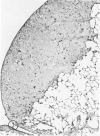
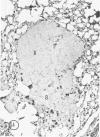
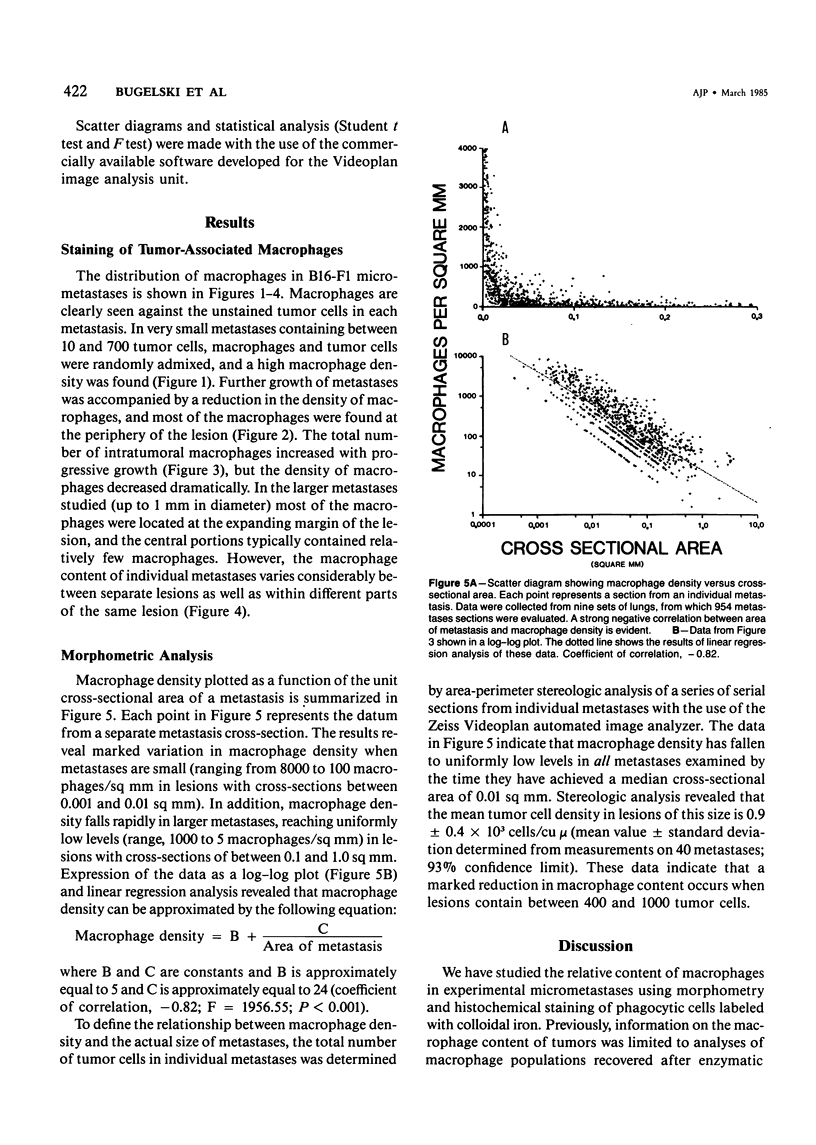
The macrophage content of experimental B16 melanoma metastases at different stages of their growth has been quantified with the use of morphometry in conjunction with a recently developed histochemical method for selectively staining intratumoral macrophages. Data are presented from analyses of 954 sections of 155 individual lung metastases, showing that the macrophage content of individual B16 melanoma lung metastases not only varies significantly but also falls dramatically once metastases contain more than 700 tumor cells. In addition to providing new information on host response reactions of micrometastases, these experiments also indicate that conclusions on intratumoral macrophages derived from studies of large primary tumors and metastases in advanced stages of growth may have little or no relevance to events in micrometastases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bugelski P. J., Kirsh R. L., Poste G. New histochemical method for measuring intratumoral macrophages and macrophage recruitment into experimental metastases. Cancer Res. 1983 Nov;43(11):5493–5501. [PubMed] [Google Scholar]
- Evans R. Macrophages and neoplasms: new insights and their implication in tumor immunobiology. Cancer Metastasis Rev. 1982;1(3):227–239. doi: 10.1007/BF00046829. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Poste G. Macrophage-mediated destruction of malignant tumor cells and new strategies for the therapy of metastatic disease. Springer Semin Immunopathol. 1982;5(2):161–174. doi: 10.1007/BF00199794. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Raz A., Fogler W. E., Kirsh R., Bugelski P., Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980 Dec;40(12):4460–4466. [PubMed] [Google Scholar]
- Fisher E. R., Gregorio R. M., Fisher B., Redmond C., Vellios F., Sommers S. C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kalish R., Brody N. I. The effects of tumor facilitating factor of B16 melanoma on the macrophage. J Invest Dermatol. 1983 Mar;80(3):162–167. doi: 10.1111/1523-1747.ep12533308. [DOI] [PubMed] [Google Scholar]
- Koestler T. P., Rieman D., Muirhead K., Greig R. G., Poste G. Identification and characterization of a monoclonal antibody to an antigen expressed on activated macrophages. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4505–4509. doi: 10.1073/pnas.81.14.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J., Bizot J., Bolesse M., Pulicani J. P. "Noir de diaminobenzidine": une nouvelle méthode histochimique de révélation du fer exogène. Histochemistry. 1980;66(3):239–244. doi: 10.1007/BF00495737. [DOI] [PubMed] [Google Scholar]
- Normann S. J., Cornelius J. Concurrent depression of tumor macrophage infiltration and systemic inflammation by progressive cancer growth. Cancer Res. 1978 Oct;38(10):3453–3459. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Kirsh R., Fogler W. E., Fidler I. J. Activation of tumoricidal properties in mouse macrophages by lymphokines encapsulated in liposomes. Cancer Res. 1979 Mar;39(3):881–892. [PubMed] [Google Scholar]
- Poste G., Kirsh R. Rapid decay of tumoricidal activity and loss of responsiveness to lymphokines in inflammatory macrophages. Cancer Res. 1979 Jul;39(7 Pt 1):2582–2590. [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Pace J. L. Evidence for mononuclear phagocytes in solid neoplasms and appraisal of their nonspecific cytotoxic capabilities. Contemp Top Immunobiol. 1980;10:143–166. doi: 10.1007/978-1-4684-3677-8_6. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Key M., Fidler I. J. Macrophage content of metastatic and nonmetastatic rodent neoplasms. J Immunol. 1981 Jun;126(6):2245–2248. [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. Detection and quantitation of macrophage infiltration into primary human tumors with the use of cell-surface markers. J Natl Cancer Inst. 1977 Oct;59(4):1081–1087. doi: 10.1093/jnci/59.4.1081. [DOI] [PubMed] [Google Scholar]
- Zietz S., Lessin S., Grattarola M., Desaive C., Nicolini C. FPi analysis. II. Use of the method to monitor the in vivo kinetics of cell populations perturbed by hydroxyurea. Cell Tissue Kinet. 1980 Sep;13(5):473–484. doi: 10.1111/j.1365-2184.1980.tb00488.x. [DOI] [PubMed] [Google Scholar]