Abstract
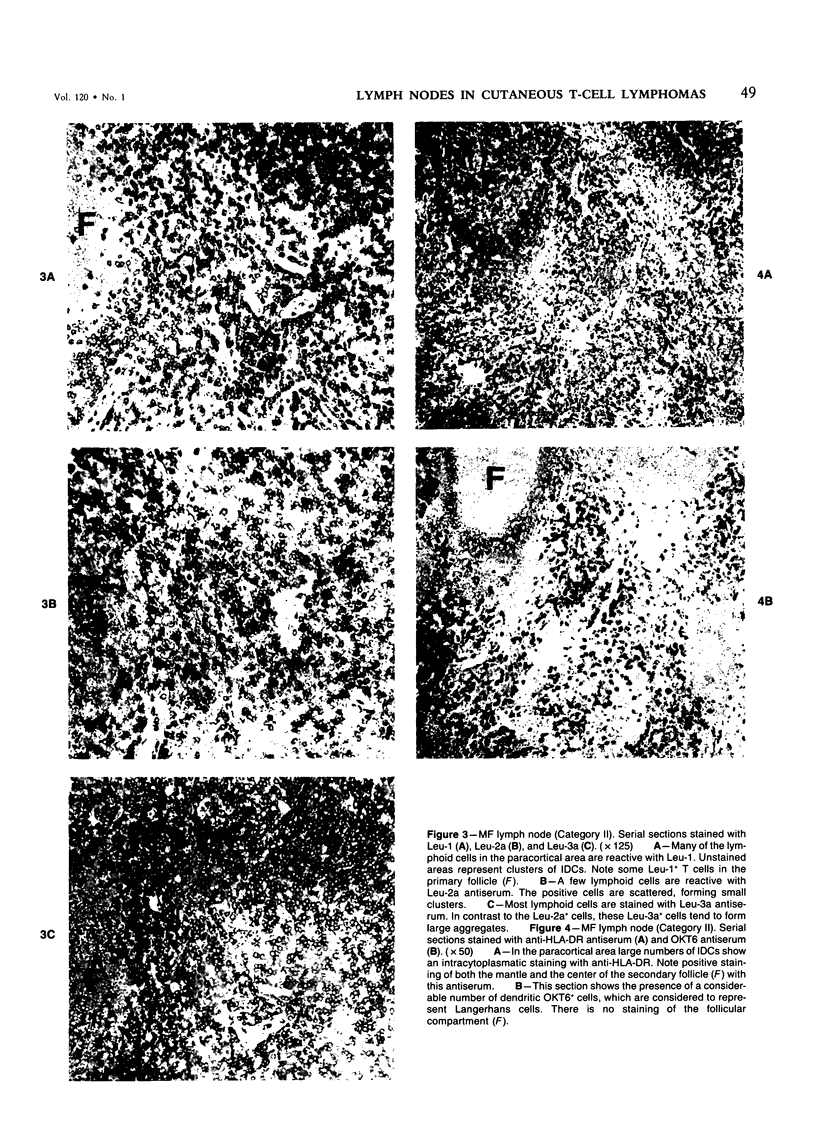
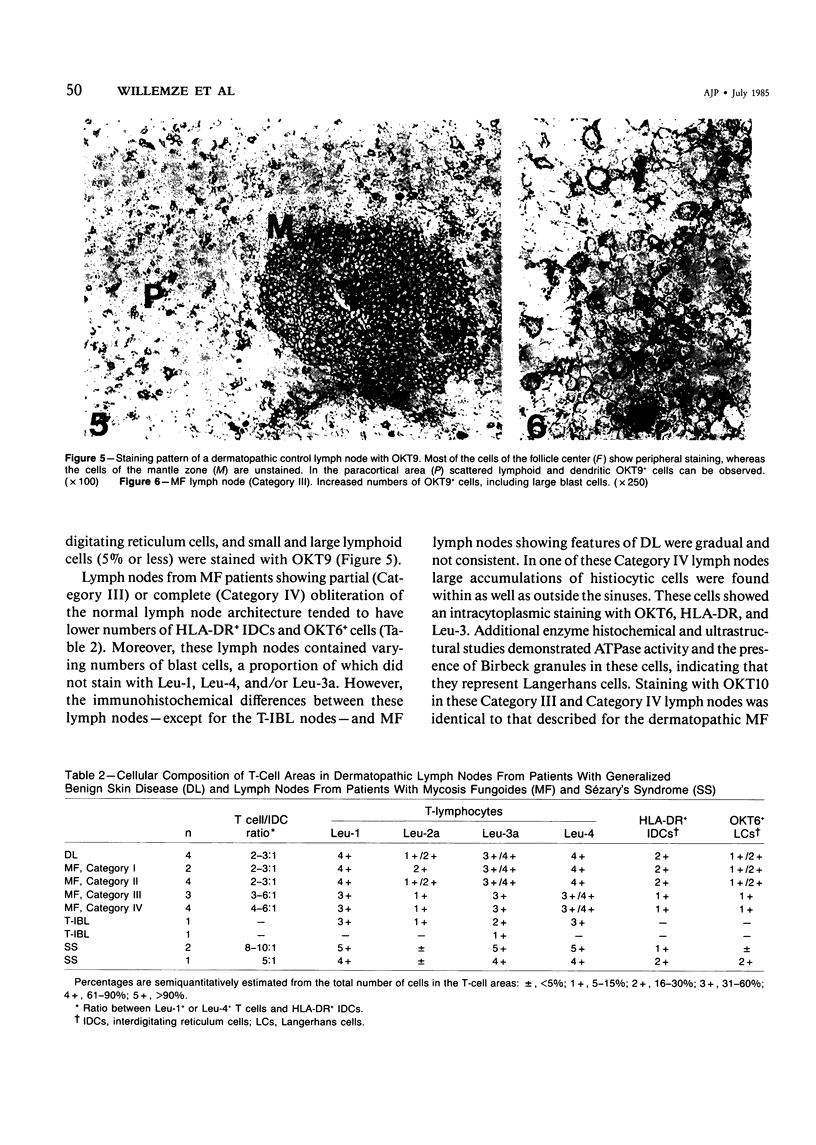
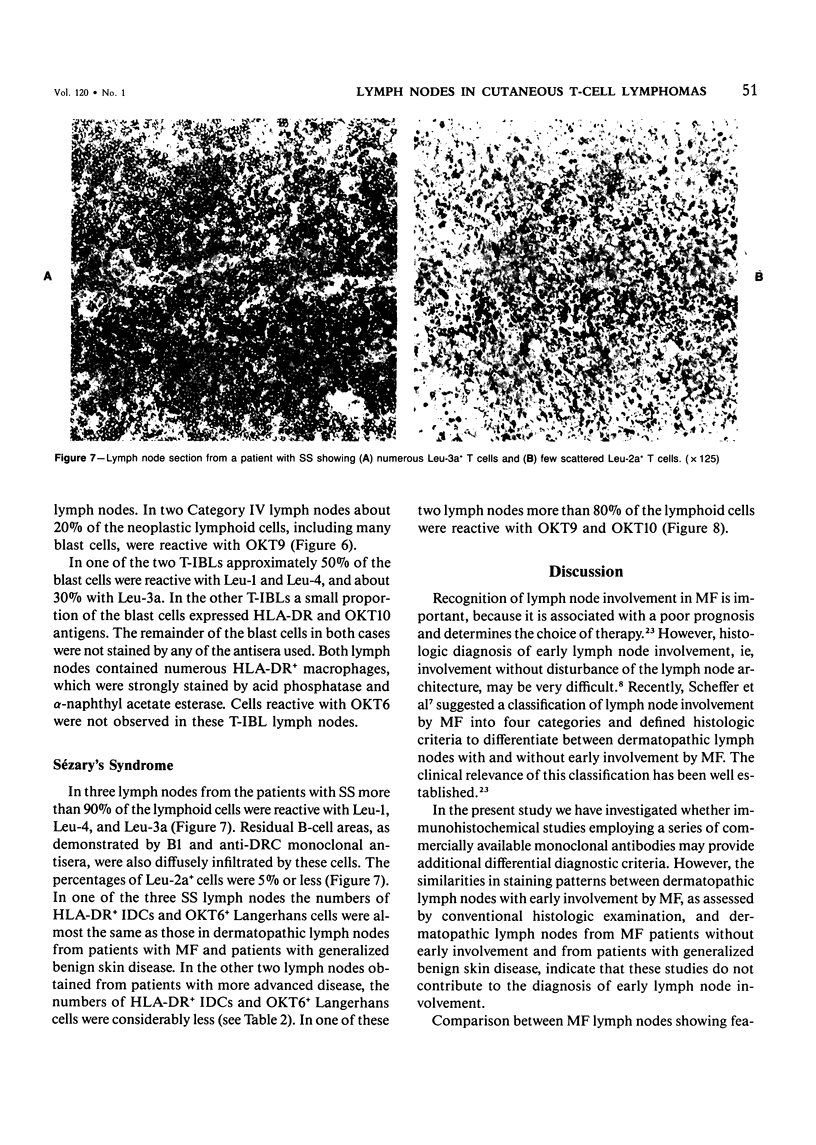
Using an indirect immunoperoxidase technique and a panel of monoclonal antibodies with well-defined specificities, the authors studied the distribution of lymphoid and nonlymphoid cells in the T-cell areas of both involved and uninvolved lymph nodes from patients with mycosis fungoides (MF) and Sézary's syndrome (SS) and dermatopathic lymph nodes from patients with generalized benign skin disease. The distribution of the different T-cell subsets, HLA-DR+ interdigitating cells and OKT6+ Langerhans cells, in the paracortical areas of MF lymph nodes showing features of dermatopathic lymphadenopathy with early involvement, as assessed after conventional histologic examination, was similar to that of dermatopathic MF lymph nodes without early involvement and lymph nodes from patients with generalized benign skin disease, indicating that these studies do not provide additional criteria for the early diagnosis of MF involvement. MF lymph nodes showing partial or complete obliteration of the normal architecture tended to have lower numbers of HLA-DR+ interdigitating cells and OKT6+ Langerhans cells, but showed increased numbers of blast cells, part of which had lost their mature helper-T-cell phenotype. These differences between the early and advanced stages of lymph node involvement by MF were analogous to those observed in the different stages of cutaneous involvement, as described previously. The lymph nodes from patients with SS were diffusely infiltrated by neoplastic T cells that had retained their mature helper-T-cell phenotype (Leu-1+, 3a+, 4+), contained very low numbers of Leu-2+ T-cells and relatively few HLA-DR+ interdigitating cells and OKT6+ Langerhans cells. These different staining patterns in MF and SS lymph nodes may reflect different pathogenetic mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Nadler L. M., Stashenko P., McCluskey R. T., Schlossman S. F. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981 Sep 1;154(3):737–749. doi: 10.1084/jem.154.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. D., Sillevis Smitt J. H., Krieg S. R., Bakker P. M., Vos G. D., van Zaane D. J. Acute disseminated histiocytosis-X: in situ immunophenotyping with monoclonal antibodies. J Cutan Pathol. 1984 Feb;11(1):59–64. doi: 10.1111/j.1600-0560.1984.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Burke J. S., Colby T. V. Dermatopathic lymphadenopathy. Comparison of cases associated and unassociated with mycosis fungoides. Am J Surg Pathol. 1981 Jun;5(4):343–352. [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeshaw J. A., Lister T. A., Stansfeld A. G., Greaves M. F. Correlation of transferrin receptor expression with histological class and outcome in non-Hodgkin lymphoma. Lancet. 1983 Mar 5;1(8323):498–501. doi: 10.1016/s0140-6736(83)92191-8. [DOI] [PubMed] [Google Scholar]
- Hamminga L., Hermans J., Noordijk E. M., Meijer C. J., Scheffer E., Van Vloten W. A. Cutaneous T-cell lymphoma: clinicopathological relationships, therapy and survival in ninety-two patients. Br J Dermatol. 1982 Aug;107(2):145–155. doi: 10.1111/j.1365-2133.1982.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Metzgar R. S., Minna J. D., Bunn P. A. Phenotypic characterization of cutaneous T-cell lymphoma. Use of monoclonal antibodies to compare with other malignant T cells. N Engl J Med. 1981 May 28;304(22):1319–1323. doi: 10.1056/NEJM198105283042202. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Halper J. P. Human T-cell malignancies: Correlative clinical, histopathologic, immunologic, and cytochemical analysis of 23 cases. Am J Pathol. 1982 Feb;106(2):187–203. [PMC free article] [PubMed] [Google Scholar]
- Kung P. C., Berger C. L., Goldstein G., LoGerfo P., Edelson R. L. Cutaneous T cell lymphoma: characterization by monoclonal antibodies. Blood. 1981 Feb;57(2):261–266. [PubMed] [Google Scholar]
- Laroche L., Bach J. F. T cell imbalance in nonleukemic and leukemic cutaneous lymphoma defined by monoclonal antibodies. Clin Immunol Immunopathol. 1981 Aug;20(2):278–284. doi: 10.1016/0090-1229(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKie R. M. Initial event in mycosis fungoides of the skin is viral infection of epidermal Langerhans cells. Lancet. 1981 Aug 8;2(8241):283–285. doi: 10.1016/s0140-6736(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Murphy G. F., Bhan A. K., Sato S., Mihm M. C., Jr, Harrist T. J. A new immunologic marker for human Langerhans cells. N Engl J Med. 1981 Mar 26;304(13):791–792. doi: 10.1056/NEJM198103263041320. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Scheffer E., Meijer C. J., Van Vloten W. A. Dermatopathic lymphadenopathy and lymph node involvement in mycosis fungoides. Cancer. 1980 Jan 1;45(1):137–148. doi: 10.1002/1097-0142(19800101)45:1<137::aid-cncr2820450124>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Janossy G., Graham-Brown R. A., Kung P. C., Goldstein G. The relationship between T lymphocyte subsets and Ia-like antigen positive nonlymphoid cells in early stages of cutaneous T cell lymphoma. J Invest Dermatol. 1982 Feb;78(2):169–176. doi: 10.1111/1523-1747.ep12506339. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Willemze R., de Graaff-Reitsma C. B., Cnossen J., Van Vloten W. A., Meijer C. J. Characterization of T-cell subpopulations in skin and peripheral blood of patients with cutaneous T-cell lymphomas and benign inflammatory dermatoses. J Invest Dermatol. 1983 Jan;80(1):60–66. doi: 10.1111/1523-1747.ep12531102. [DOI] [PubMed] [Google Scholar]
- Willemze R., van Vloten W. A., Hermans J., Damsteeg M. J., Meijer C. J. Diagnostic criteria in Sézary's syndrome: a multiparameter study of peripheral blood lymphocytes in 32 patients with erythroderma. J Invest Dermatol. 1983 Nov;81(5):392–397. doi: 10.1111/1523-1747.ep12521991. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Morhenn V. B., Butcher E. C., Kosek J. Langerhans cells react with pan-leukocyte monoclonal antibody: ultrastructural documentation using a live cell suspension immunoperoxidase technique. J Invest Dermatol. 1984 Apr;82(4):322–325. doi: 10.1111/1523-1747.ep12260618. [DOI] [PubMed] [Google Scholar]