Abstract
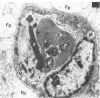
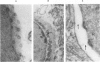
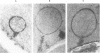
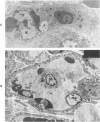
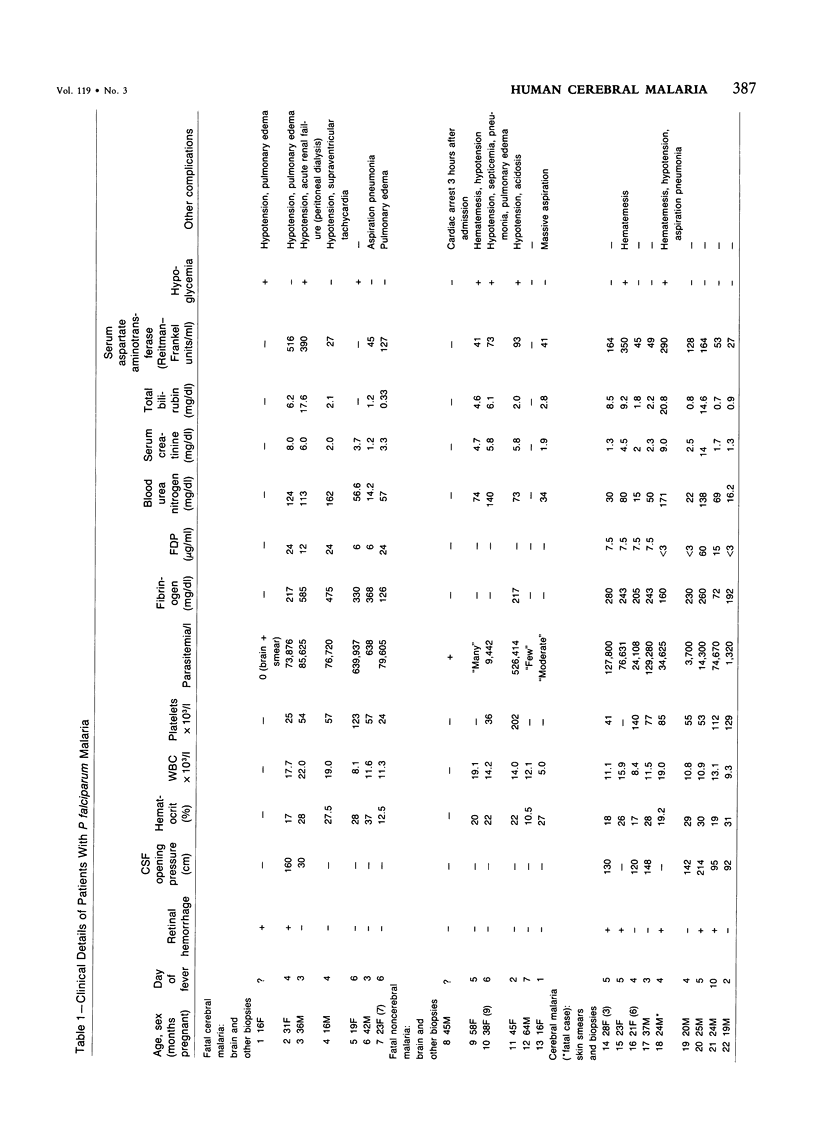
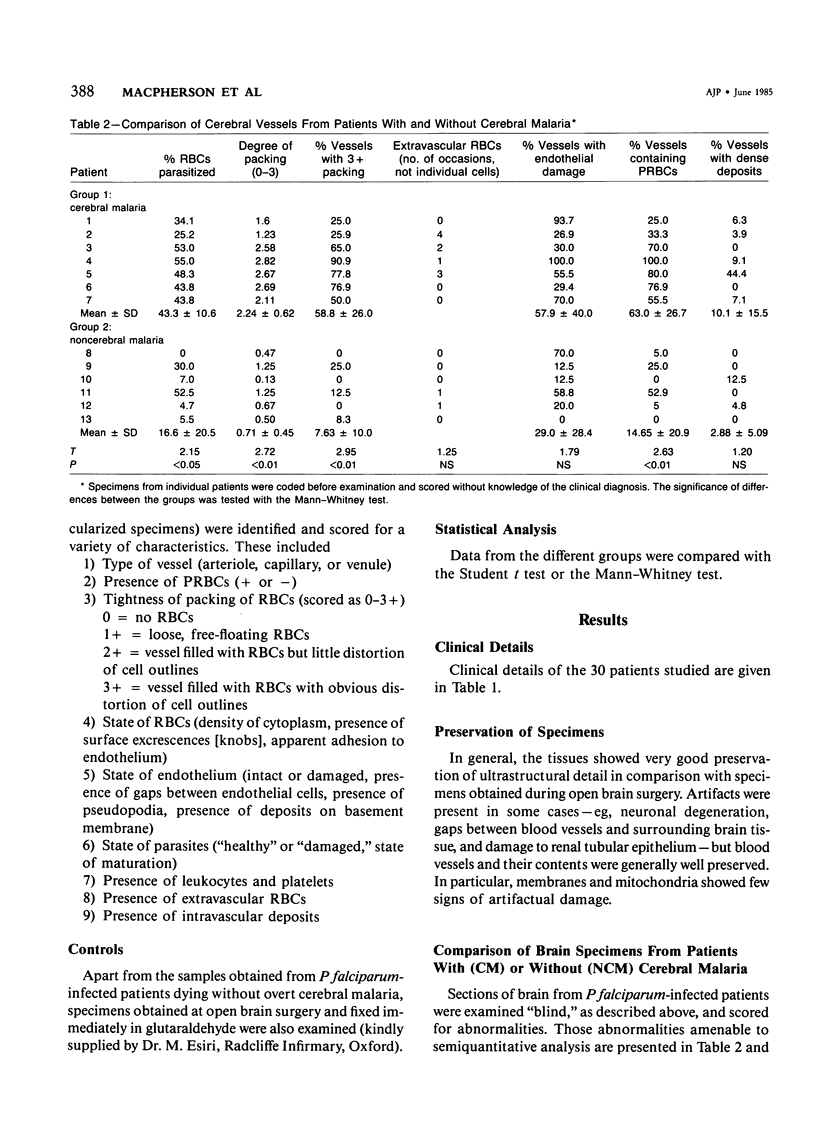
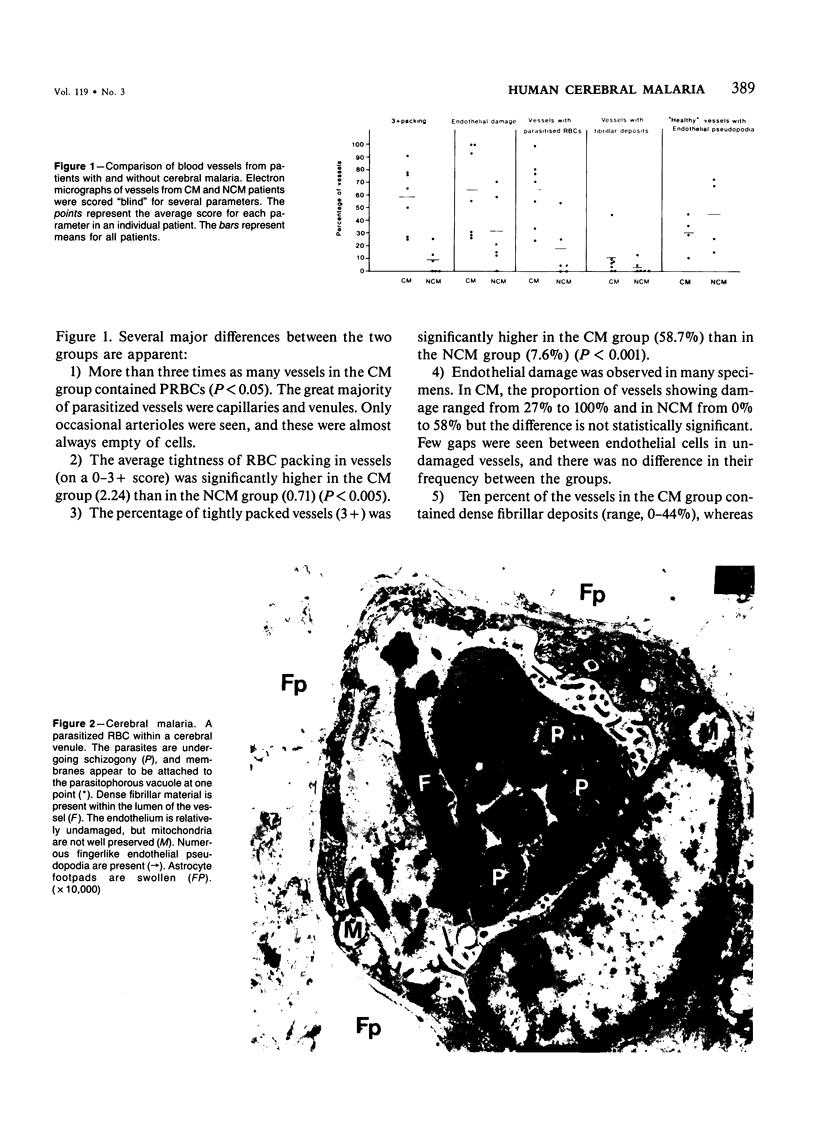
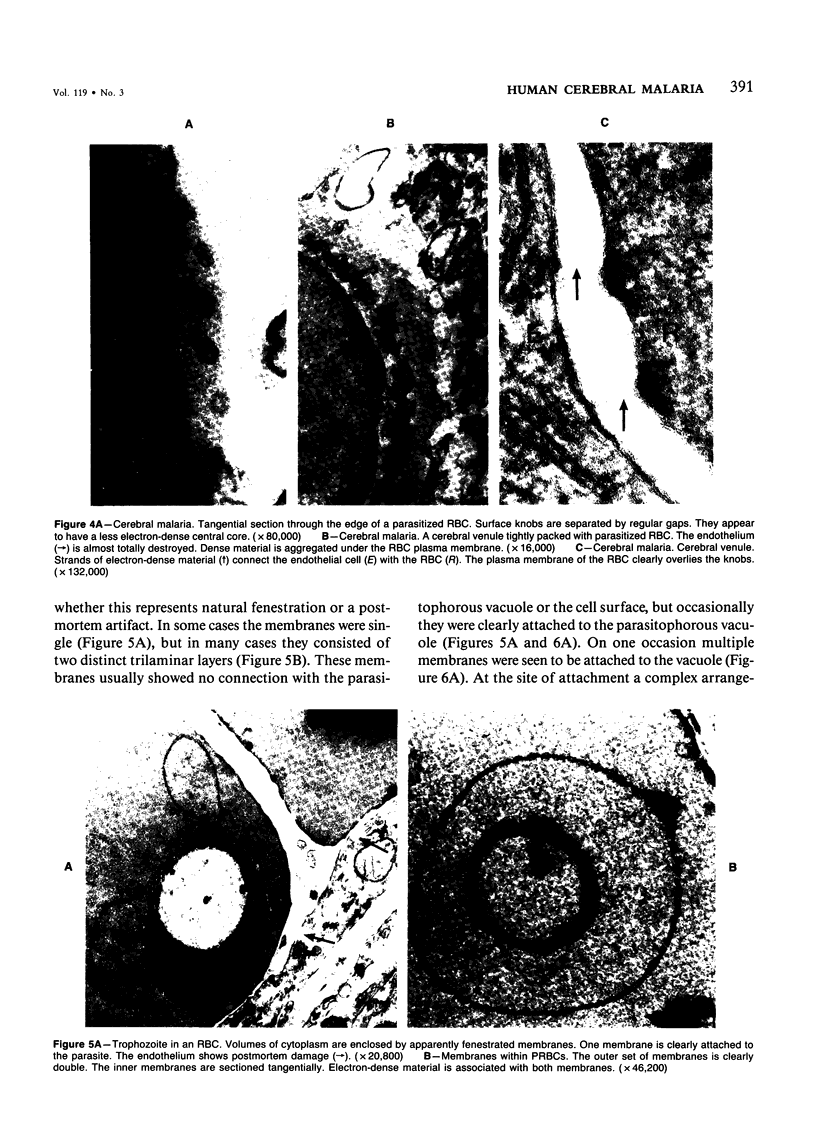
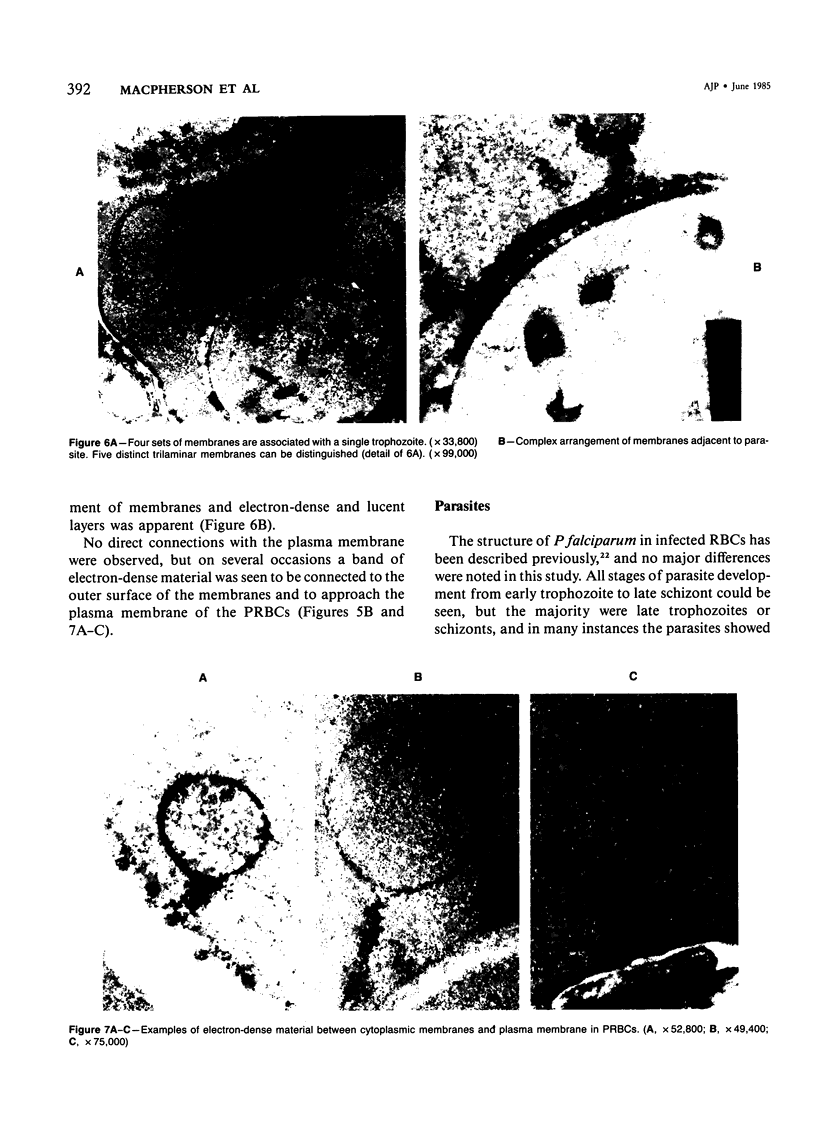
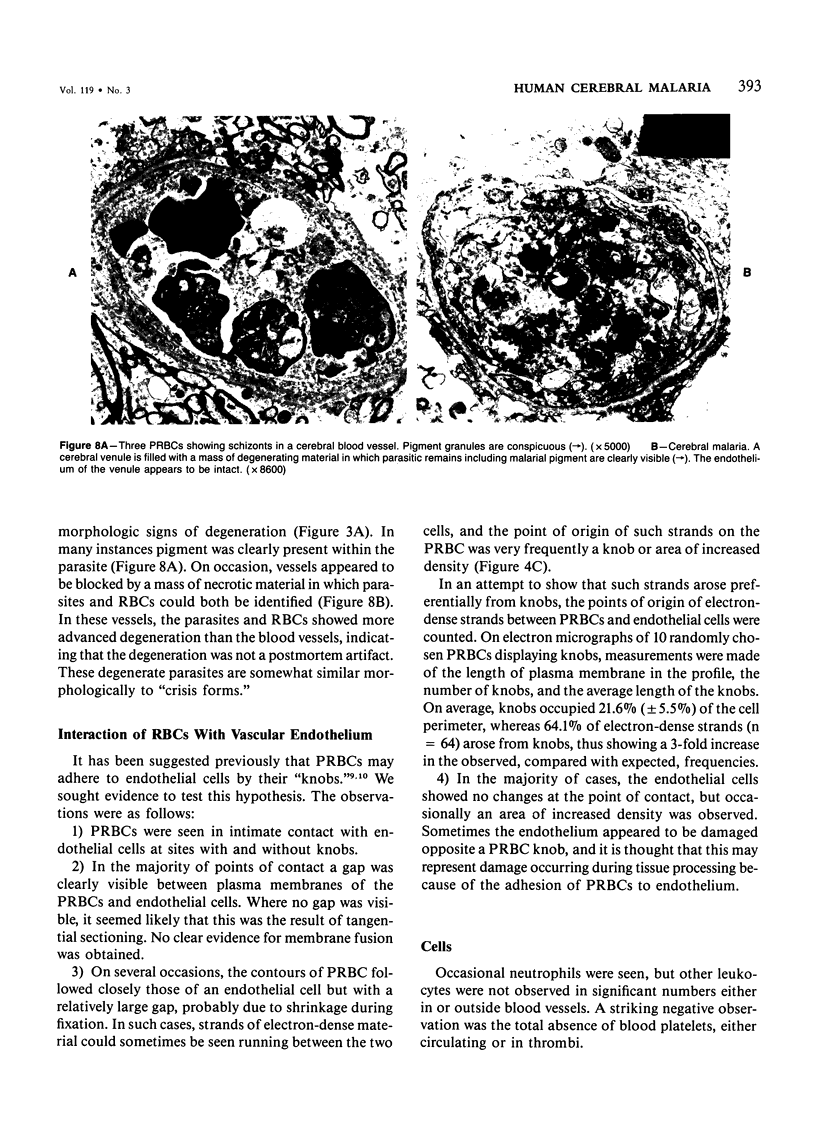
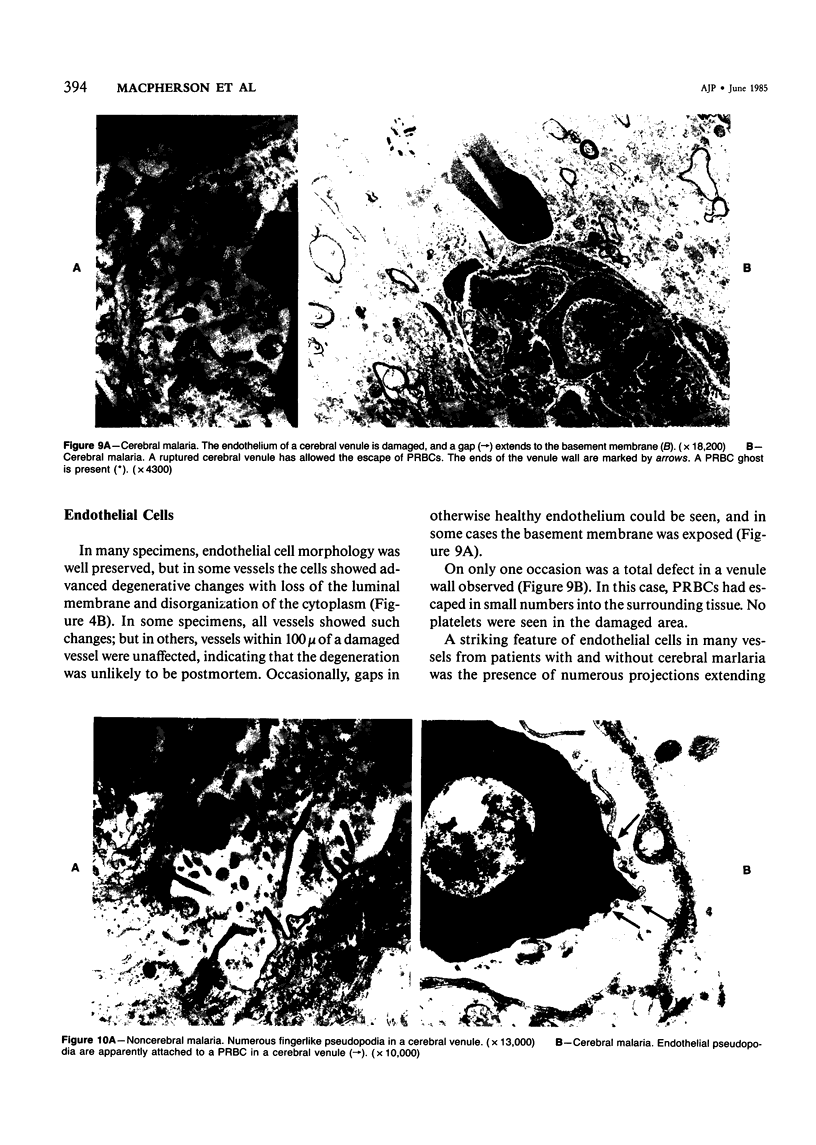
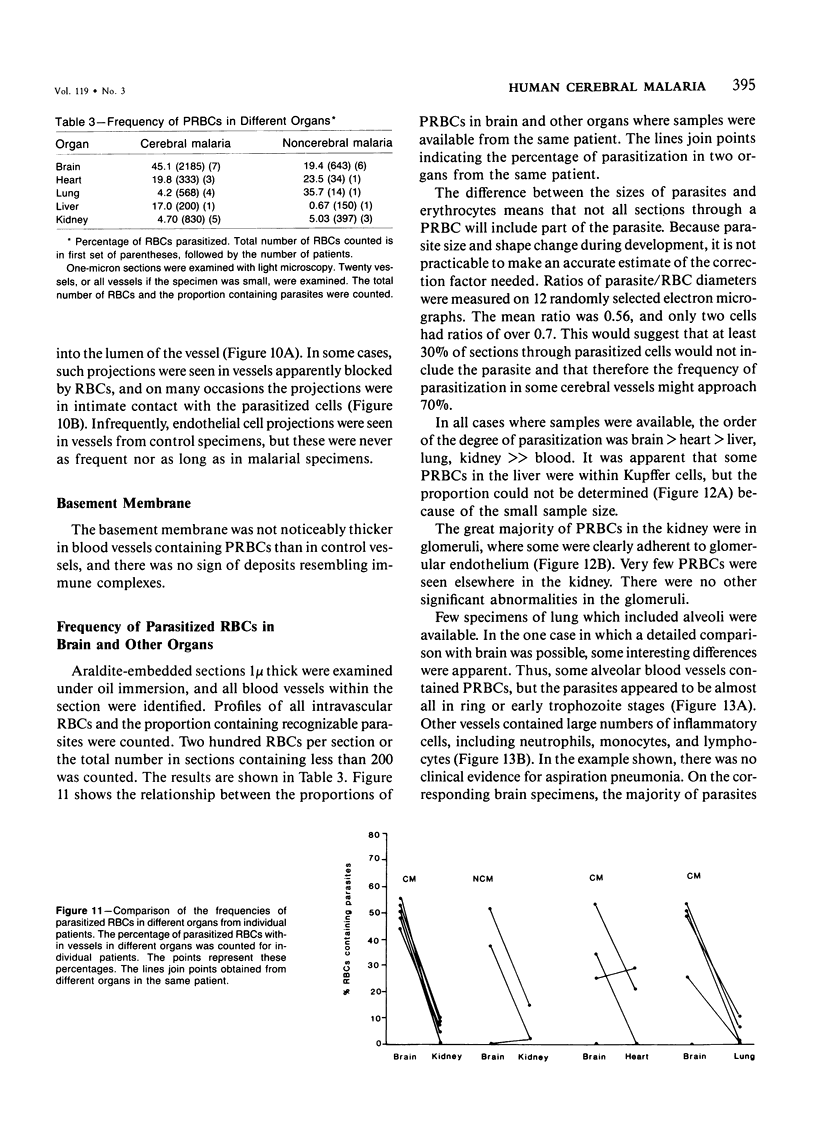
For investigation of the pathogenesis of cerebral malaria, immediate postmortem samples from brain and other tissues of patients dying with Plasmodium falciparum malaria, with (CM) or without (NCM) cerebral malaria, were processed for electron microscopy. Counts of parasitized erythrocytes (PRBCs) in cerebral and other vessels showed that the proportion of PRBCs was higher in CM than in NCM, and also that the proportion of PRBCs was higher in the brain than in other organs examined in both CM and NCM. Cerebral vessels from CM patients were more tightly packed with RBCs than those from NCM patients, but there was no significant difference in the amount or degree of endothelial damage or numbers of vessels with endothelial pseudopodia. Fibrillar (fibrin) deposits were present in a small proportion of vessels, but no thrombosis was present. There was neither acute nor chronic inflammation, and leukocytes were absent within or outside cerebral vessels. There was no immune complex deposition in cerebral vessels. Parasites in cerebral vessels were mainly trophozoites or schizonts. Occasional RBC remnants following parasite release were seen. Some parasites were degenerate, resembling crisis forms. PRBCs adhered to endothelium via surface knobs. It is concluded that there is no evidence for an inflammatory or immune pathogenesis for human cerebral malaria and that the clinical effects probably relate to anoxia and the metabolic activities of the parasites.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Daroff R. B., Deller J. J., Jr, Kastl A. J., Jr, Blocker W., Jr Cerebral malaria. JAMA. 1967 Nov 20;202(8):679–682. [PubMed] [Google Scholar]
- De Brito T., Barone A. A., Faria R. M. Human liver biopsy in P. falciparum and P. vivax malaria. A light and electron microscopy study. Virchows Arch A Pathol Pathol Anat. 1969;348(3):220–229. doi: 10.1007/BF00555648. [DOI] [PubMed] [Google Scholar]
- Dennis L. H., Eichelberger J. W., Inman M. M., Conrad M. E. Depletion of coagulation factors in drug-resistant Plasmodium falciparum malaria. Blood. 1967 May;29(5):713–721. [PubMed] [Google Scholar]
- Devakul K., Harinasuta T., Reid H. A. 125-I-labelled fibrinogen in cerebral malaria. Lancet. 1966 Oct 22;2(7469):886–888. doi: 10.1016/s0140-6736(66)91982-9. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Expression of inherited resistance to malaria in culture. Ciba Found Symp. 1983;94:196–205. doi: 10.1002/9780470715444.ch12. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gutierrez Y., Aikawa M., Fremount H. N., Sterling C. R. Experimental infection of Aotus monkeys with Plasmodium falciparum Light and electron microscopic changes. Ann Trop Med Parasitol. 1976 Mar;70(1):25–44. doi: 10.1080/00034983.1976.11687092. [DOI] [PubMed] [Google Scholar]
- Hartenbower D. L., Kantor G. L., Rosen V. J. Renal failure due to acute glomerulonephritis during falciparum malaria: case report. Mil Med. 1972 Feb;137(2):74–76. [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looareesuwan S., Warrell D. A., White N. J., Sutharasamai P., Chanthavanich P., Sundaravej K., Juel-Jensen B. E., Bunnag D., Harinasuta T. Do patients with cerebral malaria have cerebral oedema? A computed tomography study. Lancet. 1983 Feb 26;1(8322):434–437. doi: 10.1016/s0140-6736(83)91437-x. [DOI] [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- Miller L. H., Chien S., Usami S. Decreased deformability of Plasmodium coatneyi-infected red cells and its possible relation to cerebral malaria. Am J Trop Med Hyg. 1972 Mar;21(2):133–137. doi: 10.4269/ajtmh.1972.21.133. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Rest J. R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Sheehy T. W., Reba R. C. Complications of falciparum malaria and their treatment. Ann Intern Med. 1967 Apr;66(4):807–809. doi: 10.7326/0003-4819-66-4-807. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Theakston R. D. Comments on the ultrastructure of human erythrocytes infected with Plasmodium malariae. Ann Trop Med Parasitol. 1970 Sep;64(3):329–329. doi: 10.1080/00034983.1970.11686700. [DOI] [PubMed] [Google Scholar]
- Thomas J. D. Clinical and histopathological correlation of cerebral malaria. Trop Geogr Med. 1971 Sep;23(3):232–238. [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Warrell D. A., Looareesuwan S., Warrell M. J., Kasemsarn P., Intaraprasert R., Bunnag D., Harinasuta T. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N Engl J Med. 1982 Feb 11;306(6):313–319. doi: 10.1056/NEJM198202113060601. [DOI] [PubMed] [Google Scholar]
- Yoeli M., Hargreaves B. J. Brain capillary blockage produced by a virulent strain of rodent malaria. Science. 1974 May 3;184(4136):572–573. doi: 10.1126/science.184.4136.572. [DOI] [PubMed] [Google Scholar]
- Yu W. A., Yu M. C., Young P. A. Ultrastructural changes in the cerebrovascular endothelium induced by a diet high in linoleic acid and deficient in vitamin E. Exp Mol Pathol. 1974 Dec;21(3):289–299. doi: 10.1016/0014-4800(74)90096-3. [DOI] [PubMed] [Google Scholar]