Abstract
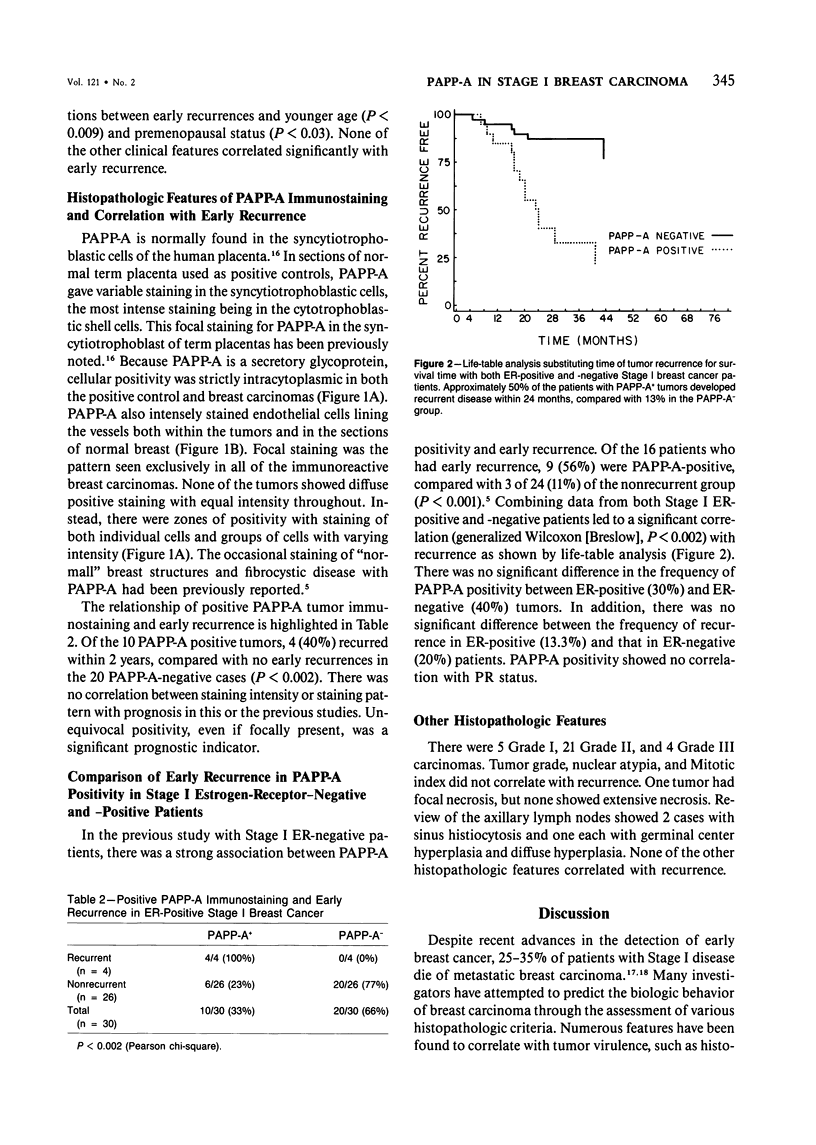
Pregnancy-associated plasma protein-A (PAPP-A) immunopositivity and extensive tumor necrosis have been shown to correlate significantly with early (less than 24 months) recurrence in patients with Stage I breast carcinoma negative for estrogen receptor (ER). In this study we have extended our analysis of Stage I disease to include 30 ER-positive cases. Twenty-five traditional clinical and pathologic features were reviewed in addition to the immunoperoxidase staining characteristics of PAPP-A for examination of the independent relationship of immunopositivity both to early recurrence and to ER and progesterone receptor (PR) status. Clinical recurrence developed in 6 of 30 (20%) patients, 4 of the 30 (13%) patients experiencing early recurrence. Pearson chi-square tests revealed significant correlations between early recurrence and PAPP-A tumor positivity (P less than 0.002), younger age (P less than 0.009), and premenopausal status (P less than 0.03). Stepwise regression analysis showed that PAPP-A-positive staining correlated independently with early recurrence. Of the 10 PAPP-A-positive tumors, 4 (40%) recurred within 2 years, compared with no early recurrences in the 20 PAPP-A-negative cases. With a comparison of frequency of PAPP-A positivity of ER-positive (30%) tumors and ER-negative tumors (40%), there was no correlation with ER or PR status. PAPP-A tumor positivity is independent of ER status and is a clinically significant independent predictor of early recurrence in both ER-positive and ER-negative Stage I patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamdal S., Børmer O., Jørgensen O., Høst H., Eliassen G., Kaalhus O., Pihl A. Estrogen receptors and long-term prognosis in breast cancer. Cancer. 1984 Jun 1;53(11):2525–2529. doi: 10.1002/1097-0142(19840601)53:11<2525::aid-cncr2820531126>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bauer T. W., O'Ceallaigh D., Eggleston J. C., Moore G. W., Baker R. R. Prognostic factors in patients with stage I, estrogen receptor-negative carcinoma of the breast. A clinicopathologic study. Cancer. 1983 Oct 15;52(8):1423–1431. doi: 10.1002/1097-0142(19831015)52:8<1423::aid-cncr2820520815>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bischof P., Martin-Du-Pan R., Lauber K., Girard J. P., Herrmann W. L., Sizonenko P. C. Human seminal plasma contains a protein that shares physicochemical, immunochemical, and immunosuppressive properties with pregnancy-associated plasma protein-A. J Clin Endocrinol Metab. 1983 Feb;56(2):359–362. doi: 10.1210/jcem-56-2-359. [DOI] [PubMed] [Google Scholar]
- Bischof P., Meisser A., Haenggeli L., Reber G., Bouvier C., Béguin F., Herrmann W. L., Sizonenko P. C. Pregnancy-associated plasma protein-A (PAPP-A) inhibits thrombin-induced coagulation of plasma. Thromb Res. 1983 Oct 1;32(1):45–55. doi: 10.1016/0049-3848(83)90153-6. [DOI] [PubMed] [Google Scholar]
- Black M. M., Barclay T. H., Hankey B. F. Prognosis in breast cancer utilizing histologic characteristics of the primary tumor. Cancer. 1975 Dec;36(6):2048–2055. doi: 10.1002/cncr.2820360919. [DOI] [PubMed] [Google Scholar]
- Boschof P., Lauber K., de Wurstemberger B., Girard J. P. Inhibition of lymphocyte transformation by pregnancy-associated plasma protein-A (PAPP-A). J Clin Lab Immunol. 1982 Jan;7(1):61–65. [PubMed] [Google Scholar]
- Bremner R. D., Nisbet A. D., Herriot R., Horne C. H., McArdle C., Crawford D., Bohn H. Detection of placental protein five (PP5) and pregnancy-specific glycoprotein (SP1) in benign and malignant breast disease. Oncodev Biol Med. 1981;2(1-2):55–62. [PubMed] [Google Scholar]
- Carter D., Pipkin R. D., Shepard R. H., Elkins R. C., Abbey H. Relationship of necrosis and tumor border to lymph node metastases and 10-year survival in carcinoma of the breast. Am J Surg Pathol. 1978 Mar;2(1):39–46. doi: 10.1097/00000478-197803000-00005. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Gregorio R. M., Fisher B., Redmond C., Vellios F., Sommers S. C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gleason D. F., Mellinger G. T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974 Jan;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- Horne C. H., Reid I. N., Milne G. D. Prognostic significance of inappropriate production of pregnancy proteins by breast cancers. Lancet. 1976 Aug 7;2(7980):279–282. doi: 10.1016/s0140-6736(76)90731-5. [DOI] [PubMed] [Google Scholar]
- Hunter R. L., Ferguson D. J., Coppleson L. W. Survival with mammary cancer related to the interaction of germinal center hyperplasia and sinus histiocytosis in axillary and internal mammary lymph nodes. Cancer. 1975 Aug;36(2):528–539. doi: 10.1002/1097-0142(197508)36:2<528::aid-cncr2820360232>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hutter R. V. The influence of pathologic factors on breast cancer management. Cancer. 1980 Aug 15;46(4 Suppl):961–976. doi: 10.1002/1097-0142(19800815)46:4+<961::aid-cncr2820461319>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Johnson R. B., Jr, Nakamura R. M., Libby R. M. Simplified Scatchard-plot assay for estrogen receptor in human breast tumor. Clin Chem. 1975 Nov;21(12):1725–1730. [PubMed] [Google Scholar]
- Knight W. A., Livingston R. B., Gregory E. J., McGuire W. L. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977 Dec;37(12):4669–4671. [PubMed] [Google Scholar]
- Kuhajda F. P., Bohn H., Mendelsohn G. Pregnancy-specific beta-1 glycoprotein (SP-1) in breast carcinoma. Pathologic and clinical considerations. Cancer. 1984 Oct 1;54(7):1392–1396. doi: 10.1002/1097-0142(19841001)54:7<1392::aid-cncr2820540727>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kuhajda F. P., Offutt L. E., Mendelsohn G. The distribution of carcinoembryonic antigen in breast carcinoma. Diagnostic and prognostic implications. Cancer. 1983 Oct 1;52(7):1257–1264. doi: 10.1002/1097-0142(19831001)52:7<1257::aid-cncr2820520721>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lin T. M., Galbert S. P., Kiefer D., Spellacy W. N., Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol. 1974 Jan 15;118(2):223–236. doi: 10.1016/0002-9378(74)90553-5. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Maynard P. V., Davies C. J., Blamey R. W., Elston C. W., Johnson J., Griffiths K. Relationship between oestrogen-receptor content and histological grade in human primary breast tumours. Br J Cancer. 1978 Dec;38(6):745–748. doi: 10.1038/bjc.1978.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. A., Hsi B., Faulk W. P., Klopper A., Thomson R. Immunological studies of the human placenta: functional and morphological analysis of pregnancy-associated plasma protein A (PAPP-A). Immunology. 1981 Nov;44(3):577–583. [PMC free article] [PubMed] [Google Scholar]
- Meyer J. S., Friedman E., McCrate M. M., Bauer W. C. Prediction of early course of breast carcinoma by thymidine labeling. Cancer. 1983 May 15;51(10):1879–1886. doi: 10.1002/1097-0142(19830515)51:10<1879::aid-cncr2820511021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pledger D. R., Belfield A. An ELISA for pregnancy-associated plasma protein A. Ann Clin Biochem. 1983 Jul;20(Pt 4):236–240. doi: 10.1177/000456328302000410. [DOI] [PubMed] [Google Scholar]
- Sjöberg J., Wahlström T., Seppä M., Rutanen E. M., Koistinen R., Koskimies A. I., Tenhunen A., Sinosich M. J., Grudzinskas J. G. Hyperstimulated human preovulatory follicular fluid, luteinized cells of unruptured follicles, and corpus luteum contain pregnancy-associated plasma protein A (PAPP-A). Fertil Steril. 1984 Apr;41(4):551–557. doi: 10.1016/s0015-0282(16)47776-9. [DOI] [PubMed] [Google Scholar]



