Abstract
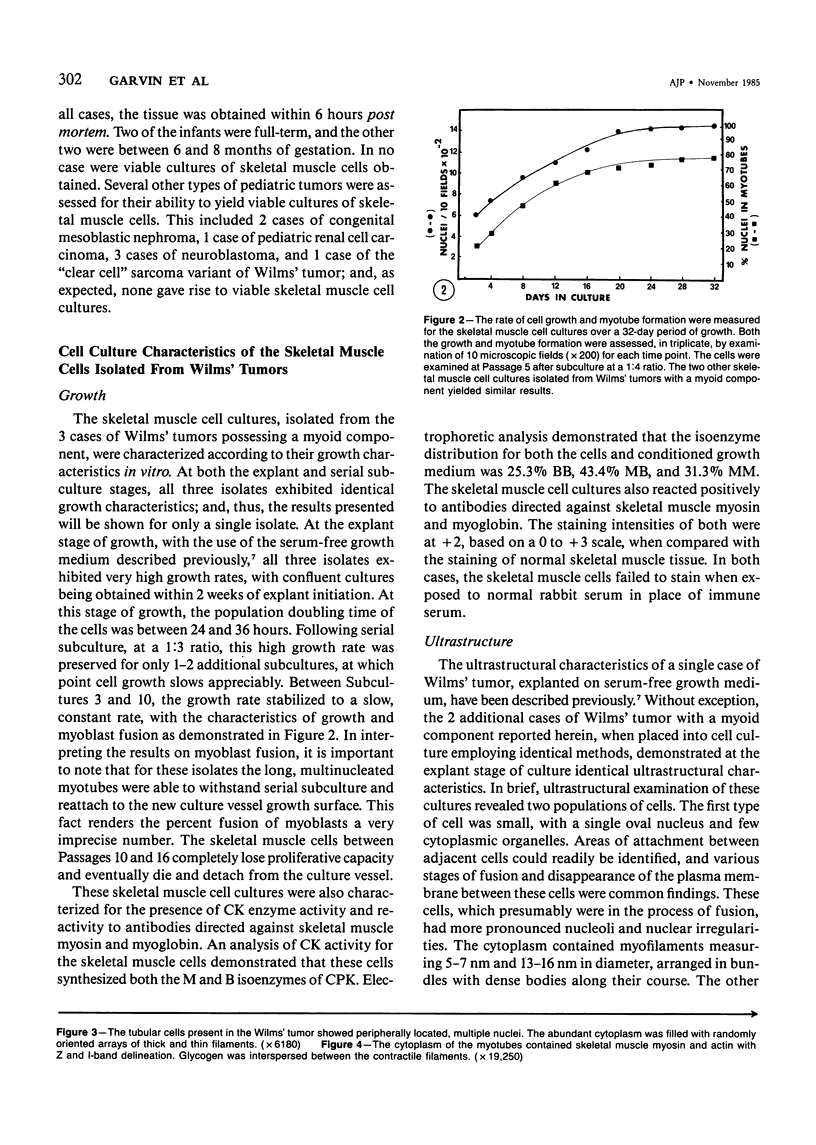
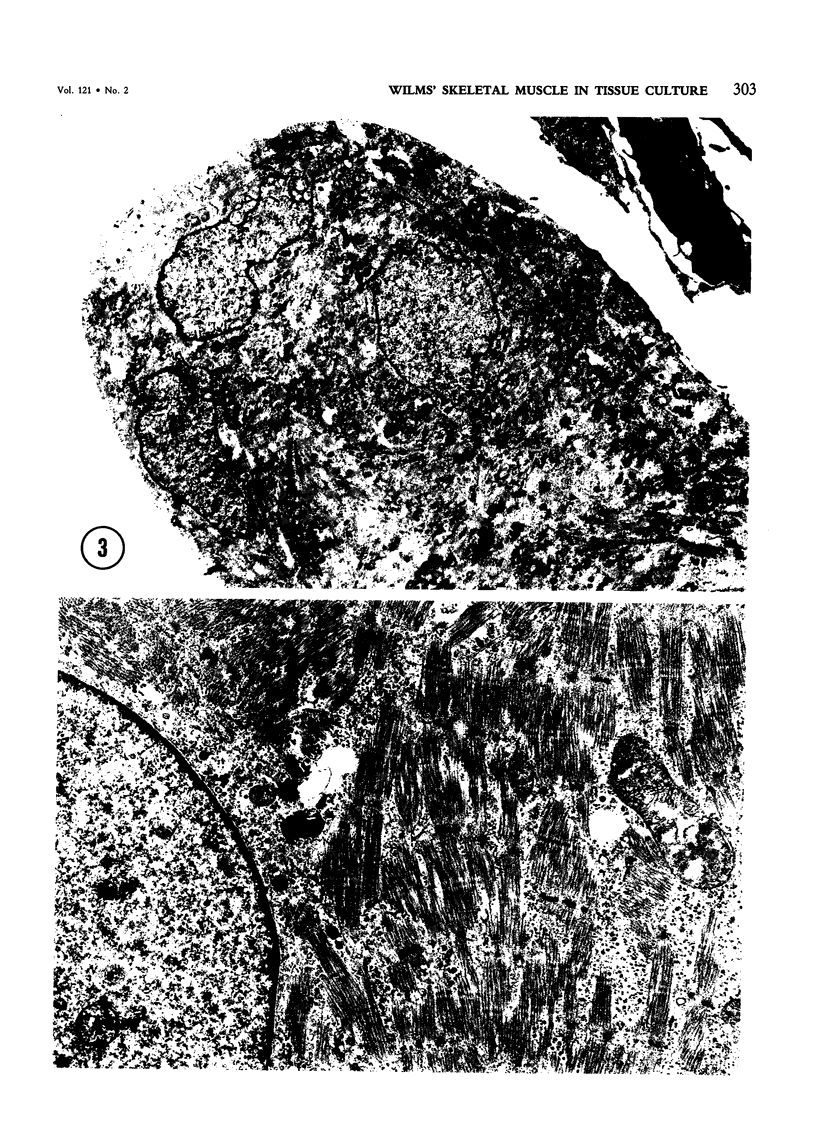
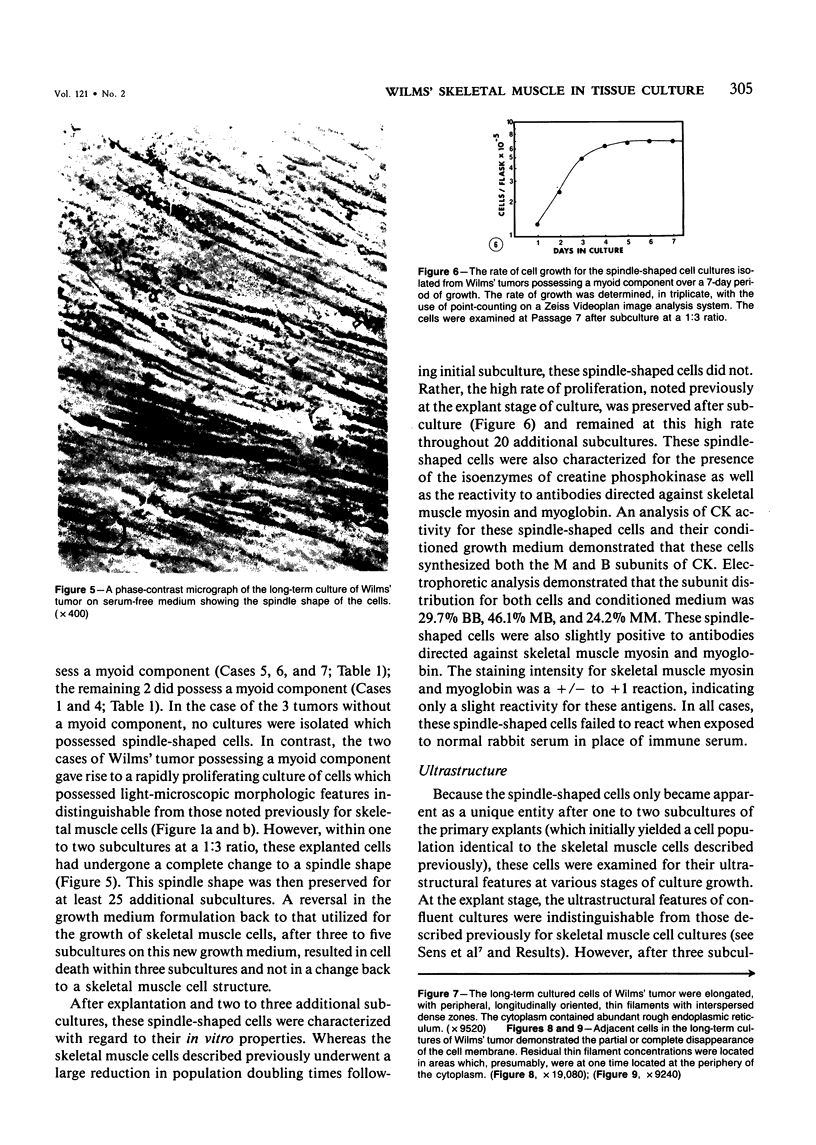
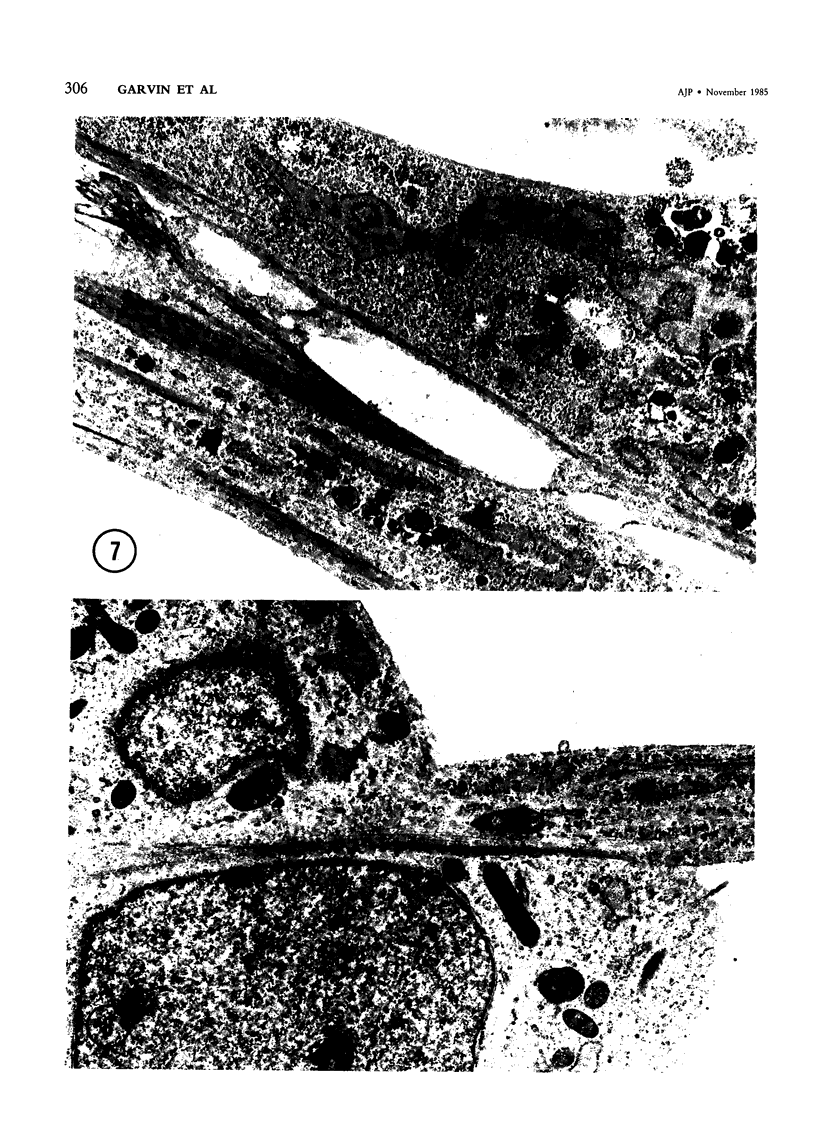
Skeletal muscle differentiation within a Wilms' tumor is a well-documented histopathologic entity thought to occur at a relatively low incidence and influence prognosis. A serum-free hormonally defined growth medium has been developed, allowing the long-term growth of the skeletal muscle component of Wilms' tumors. Eight Wilms' tumors have been grown under these conditions. Three cases grew a homogeneous population of cells which ultrastructurally displayed all stages of myogenesis through myotubule formation. They also possessed immunoreactivity for skeletal muscle myosin and myoglobin and synthesized the M and B subunits of creatine kinase. Of interest was the finding that the ability to yield skeletal muscle cultures was limited to those cases which exhibited skeletal muscle fibers in vivo. This technique is also a very sensitive marker for identifying Wilms' tumors possessing a myoid component. A second serum-free hormonally defined medium has also been developed that supports the long-term culture of a unique cell type from Wilms' tumors which contain a myoid component. These cells are spindle-shaped and exhibit all of the characteristics of early myoblasts.
Full text
PDF


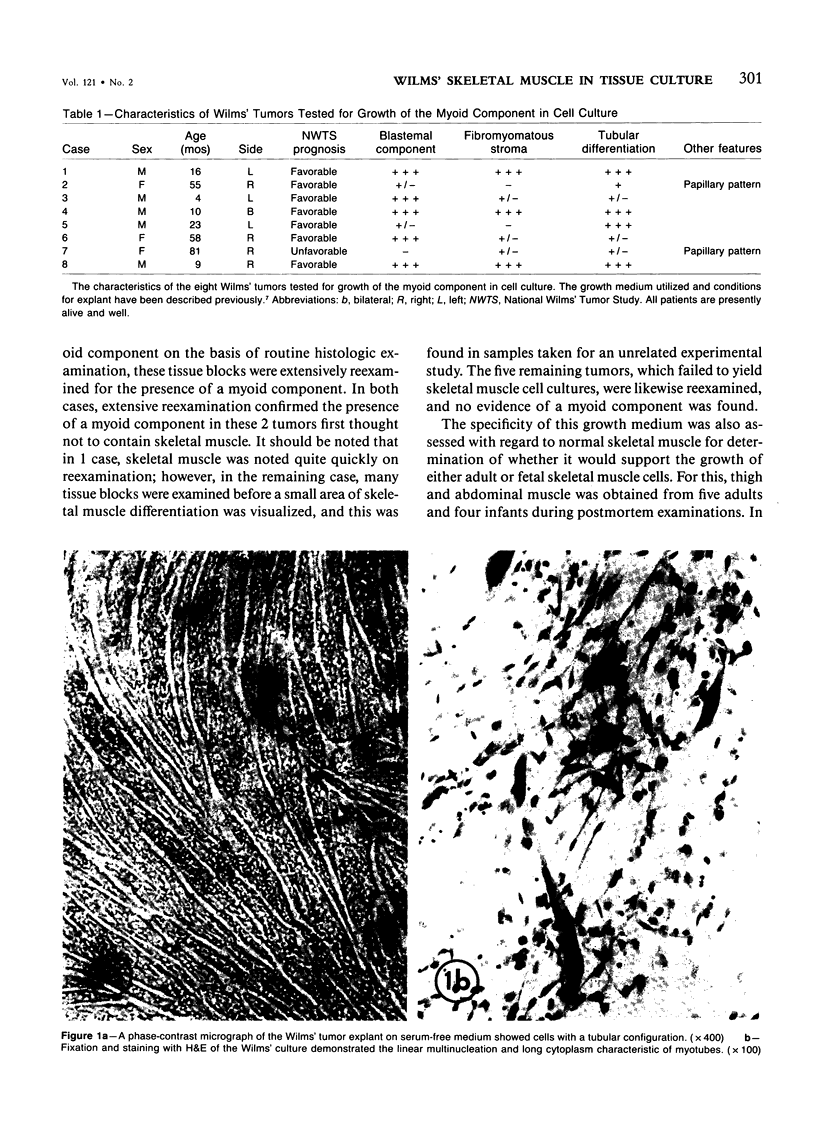

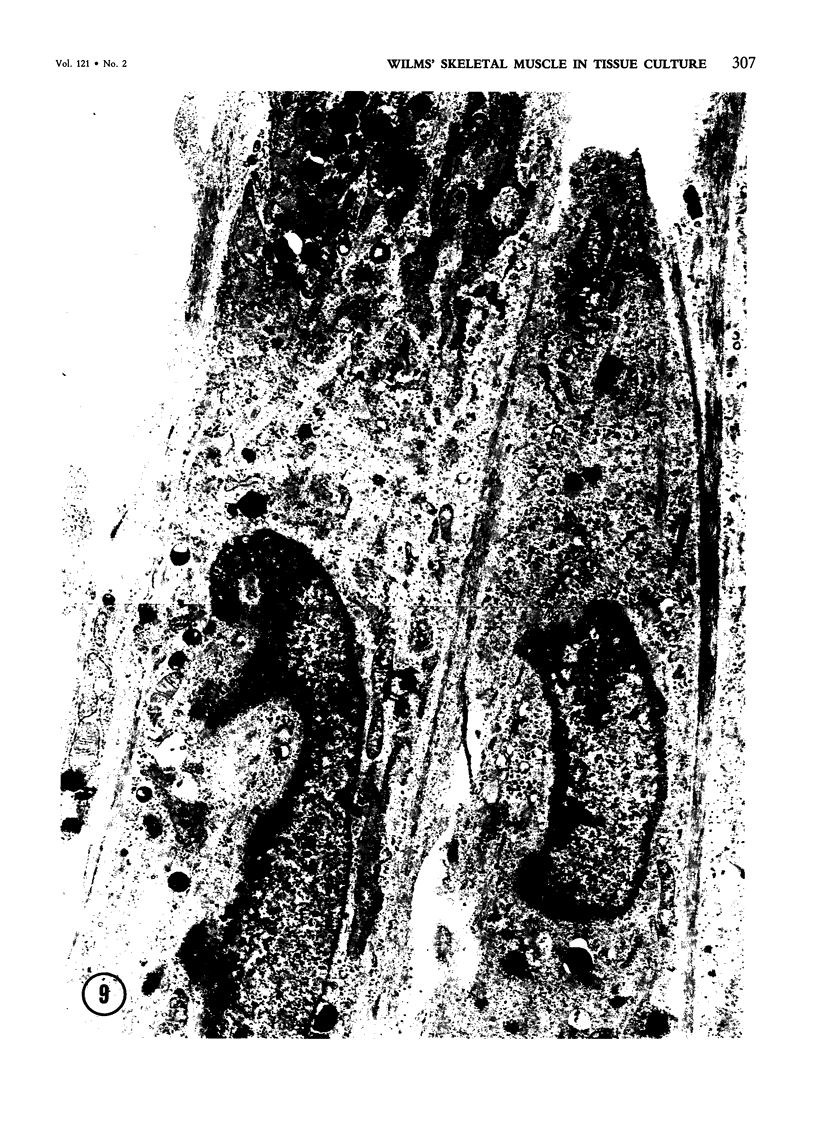



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow N. Perspectives on the statistician's role in cooperative clinical research. Cancer. 1978 Jan;41(1):326–332. doi: 10.1002/1097-0142(197801)41:1<326::aid-cncr2820410143>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z. Insulin acts as a somatomedin analog in stimulating myoblast growth in serum-free medium. In Vitro. 1981 Sep;17(9):763–768. doi: 10.1007/BF02618442. [DOI] [PubMed] [Google Scholar]
- Garvin A. J., Drew S. W., Hintz D. S., Sens D. A. Tissue culture as a diagnostic technique for soft-tissue sarcoma of childhood. Arch Pathol Lab Med. 1984 Apr;108(4):308–310. [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W., Ugarte N. Rhabdomyogenesis in renal neoplasia of childhood. Am J Surg Pathol. 1981 Sep;5(6):525–532. doi: 10.1097/00000478-198109000-00001. [DOI] [PubMed] [Google Scholar]
- Lemerle J., Tournade M. F., Marchant R. G., Flamant R., Sarrazin D., Flamant F., Lemerle M., Jundt S., Zucker J. M., Schweisguth O. Wilms' tumor: natural history and prognostic factors: a retrospective study of 248 cases treated at the Institut Gustave-Roussy 1952-1967. Cancer. 1976 May;37(5):2557–2566. doi: 10.1002/1097-0142(197605)37:5<2557::aid-cncr2820370549>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschika S. D. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981 Aug;86(1):19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Roelofs R. I., Engel W. K. Ultrastructural development of explanted human skeletal muscle in tissue culture. J Neuropathol Exp Neurol. 1972 Jul;31(3):433–436. doi: 10.1097/00005072-197207000-00003. [DOI] [PubMed] [Google Scholar]
- Nuss D., Duursma J., Pudifin D. J., Pirie D., Mickel R. E. Cultural characteristics of mesoblastic nephromas. J Pediatr Surg. 1977 Aug;12(4):553–561. doi: 10.1016/0022-3468(77)90196-8. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Schlessinger J., Yamada K. M. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979 Sep;122(2):317–326. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Robinson K. M., Gregory M. A., Nuss D., Kasvalu K. M. Growth patterns, ultrastructure, and chromosomal constitution of normal kidney and nephroblastoma cells in short-term culture. J Pediatr Surg. 1980 Jun;15(3):297–302. doi: 10.1016/s0022-3468(80)80141-2. [DOI] [PubMed] [Google Scholar]
- Rousseau-Merck M. F., Lombard M. N., Nezelof C., Mouly H. Limitation of the potentialities of nephroblastoma differentiation in vitro. Eur J Cancer. 1977 Feb;13(2):163–170. doi: 10.1016/0014-2964(77)90195-5. [DOI] [PubMed] [Google Scholar]
- Rousseau M. F., Nabarra B., Nezelof C. Behaviour of Wilms tumour and normal metanephros in organ culture. Eur J Cancer. 1974 Aug;10(8):461–466. doi: 10.1016/0014-2964(74)90066-8. [DOI] [PubMed] [Google Scholar]
- Seidal T., Kindblom L. G. The ultrastructure of alveolar and embryonal rhabdomyosarcoma. A correlative light and electron microscopic study of 17 cases. Acta Pathol Microbiol Immunol Scand A. 1984 Jul;92(4):231–248. [PubMed] [Google Scholar]
- Sens M. A., Garvin A. J., Drew S., Smith C. D., Othersen H. B., Jr, Sens D. A. Skeletal muscle differentiation in Wilms' tumor. Antibody identification and explant culture. Arch Pathol Lab Med. 1984 Jan;108(1):58–62. [PubMed] [Google Scholar]
- Szasz G., Gruber W., Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem. 1976 May;22(5):650–656. [PubMed] [Google Scholar]
- Tsokos M., Howard R., Costa J. Immunohistochemical study of alveolar and embryonal rhabdomyosarcoma. Lab Invest. 1983 Feb;48(2):148–155. [PubMed] [Google Scholar]
- Waghe M., Kumar S. Studies of hydrolytic enzymes and isoenzymes of normal and neoplastic childhood renal tissues and their tissue cultured cells. Br J Urol. 1977 Jun;49(3):189–194. doi: 10.1111/j.1464-410x.1977.tb04100.x. [DOI] [PubMed] [Google Scholar]
- Wahrmann J. P., Delain D., Bournoutian C., Macieira-Coelho A. Modulation of differentiation in vitro. I. Influence of the attachment surface on myogenesis. In Vitro. 1981 Sep;17(9):752–762. doi: 10.1007/BF02618441. [DOI] [PubMed] [Google Scholar]
- Wigger H. J. Fetal rhabdomyomatous nephroblastoma-a variant of Wilms' tumor. Hum Pathol. 1976 Nov;7(6):613–623. doi: 10.1016/s0046-8177(76)80075-5. [DOI] [PubMed] [Google Scholar]