Abstract
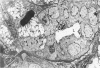
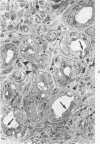
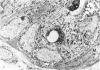
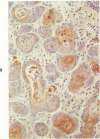
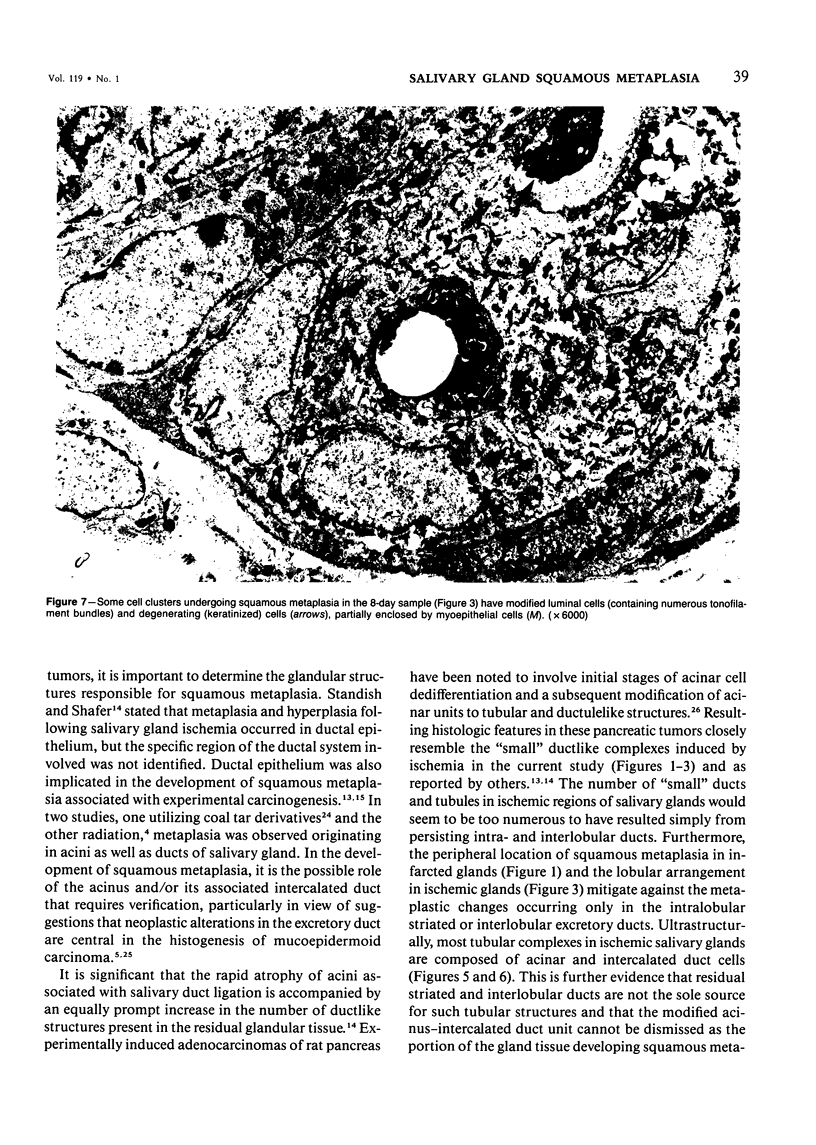
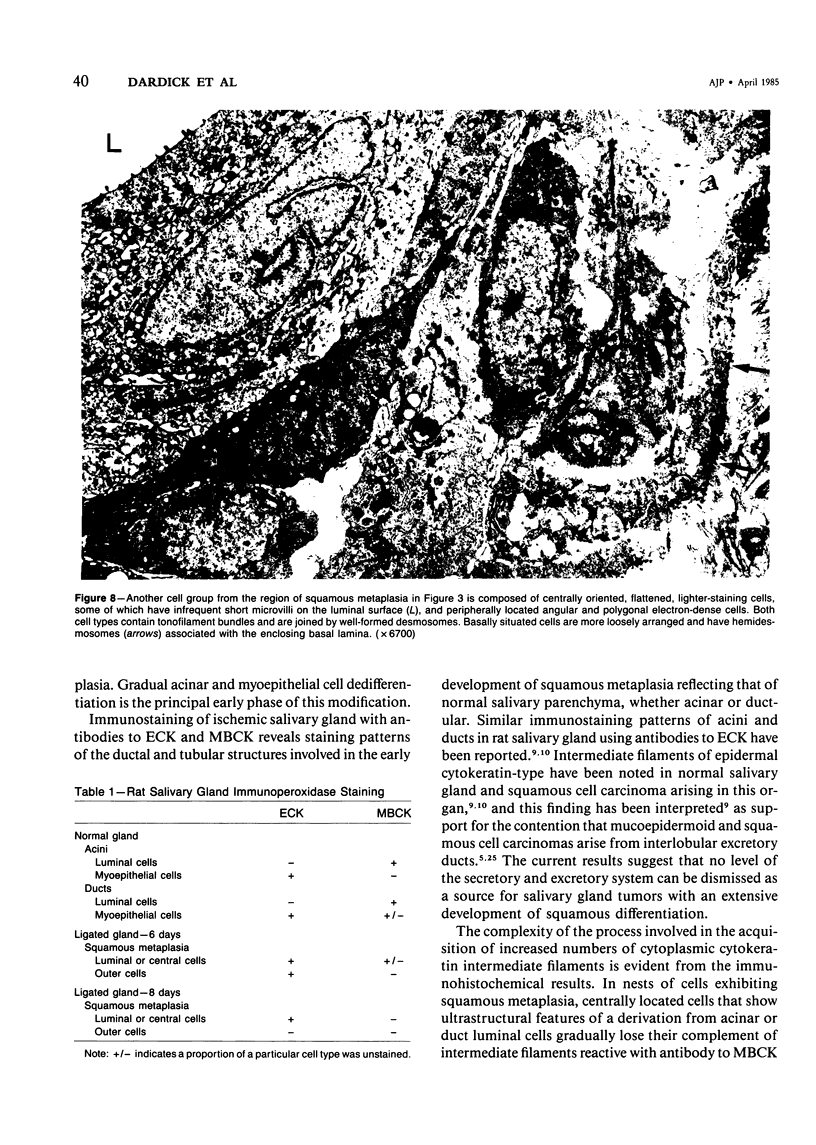
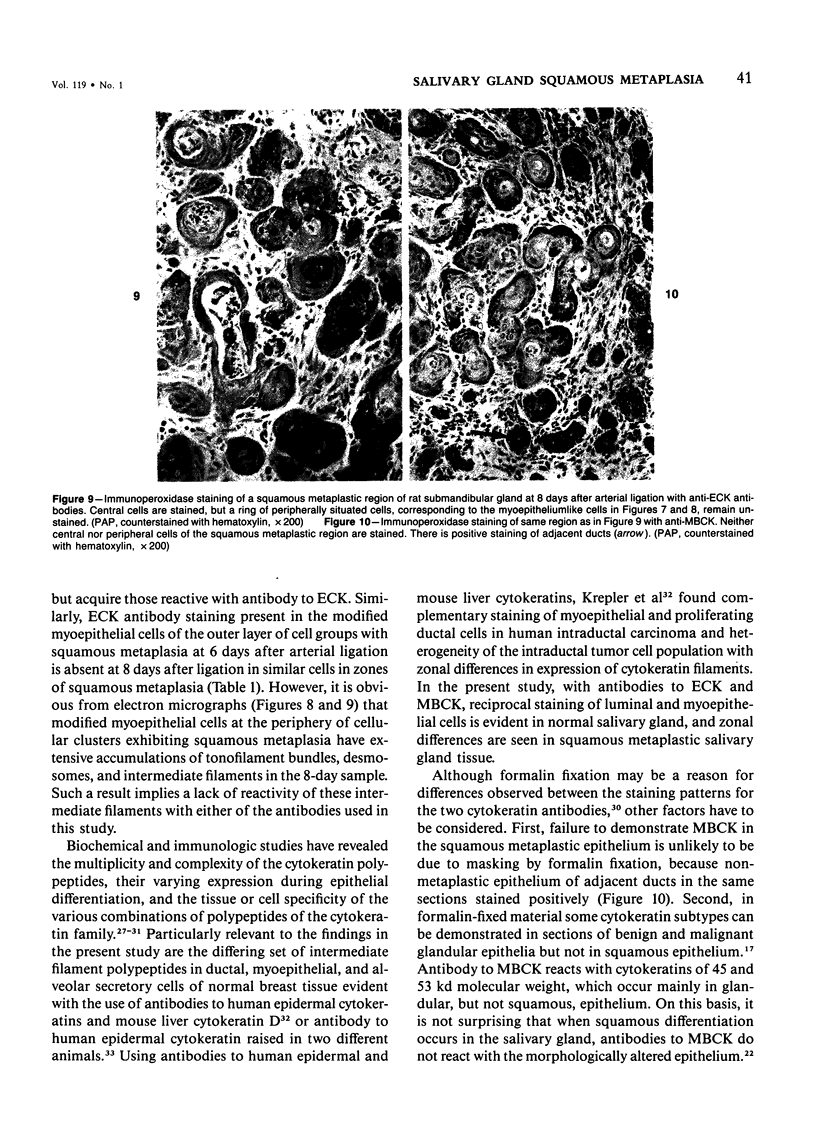
Squamous metaplasia is not an uncommon feature of a number of salivary gland lesions. Arterial ligation of rat submandibular and sublingual salivary glands was used for study of the processes and cell types involved in the development of the squamous metaplasia that occurs in ischemic and infarcted portions of gland parenchyma 6 to 8 days following vessel ligation. Light and electron micrographs show that the principal portion of salivary gland tissue undergoing squamous metaplasia is the acinar-intercalated duct cell complex. Early stages of this process involve a gradual dedifferentiation of acinar cells and hyperplasia of acinar, duct luminal cells, and myoepithelium. Subsequently, both luminal and myoepithelial cells have increasing accumulation of tonofilaments and formation of desmosomes, and centrally located cells may undergo keratinization. Immunohistochemical staining of ischemic salivary gland tissue with developing squamous metaplasia was performed with the use of rabbit antisera to human epidermal and Mallory body cytokeratins. The two antisera gave complementary patterns in normal acini and ducts, with antibody to epidermal cytokeratin (ECK) staining only myoepithelial cells and antibody to Mallory body cytokeratin (MBCK) staining mainly luminal epithelial cells. In early phases of squamous metaplasia (6 days after ligation), antibody to ECK stained central and peripheral (myoepithelial) cells, but by 8 days after ligation only central cells were stained. At 6 days after ligation, a proportion of central cells in squamoid clusters stained with antibody to MBCK, and myoepithelial cells were unstained. By 8 days after arterial ligation, cell clusters exhibiting squamous metaplasia were completely unstained with antibody to MBCK, despite the presence ultrastructurally of numerous tonofilament bundles in both types of cells forming these clusters. The propensity for squamous alteration of acinar-intercalated duct complexes has important connotations for salivary gland tumors such as pleomorphic adenoma and mucoepidermoid carcinoma.
Full text
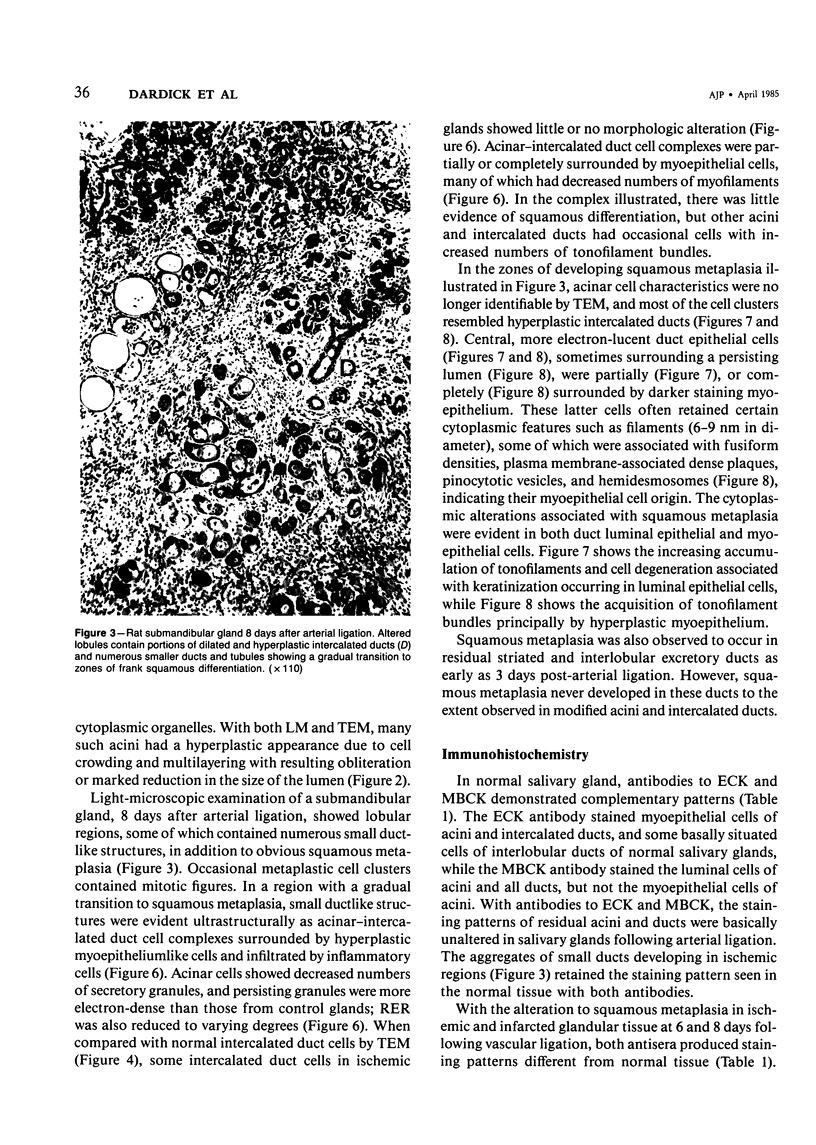
PDF


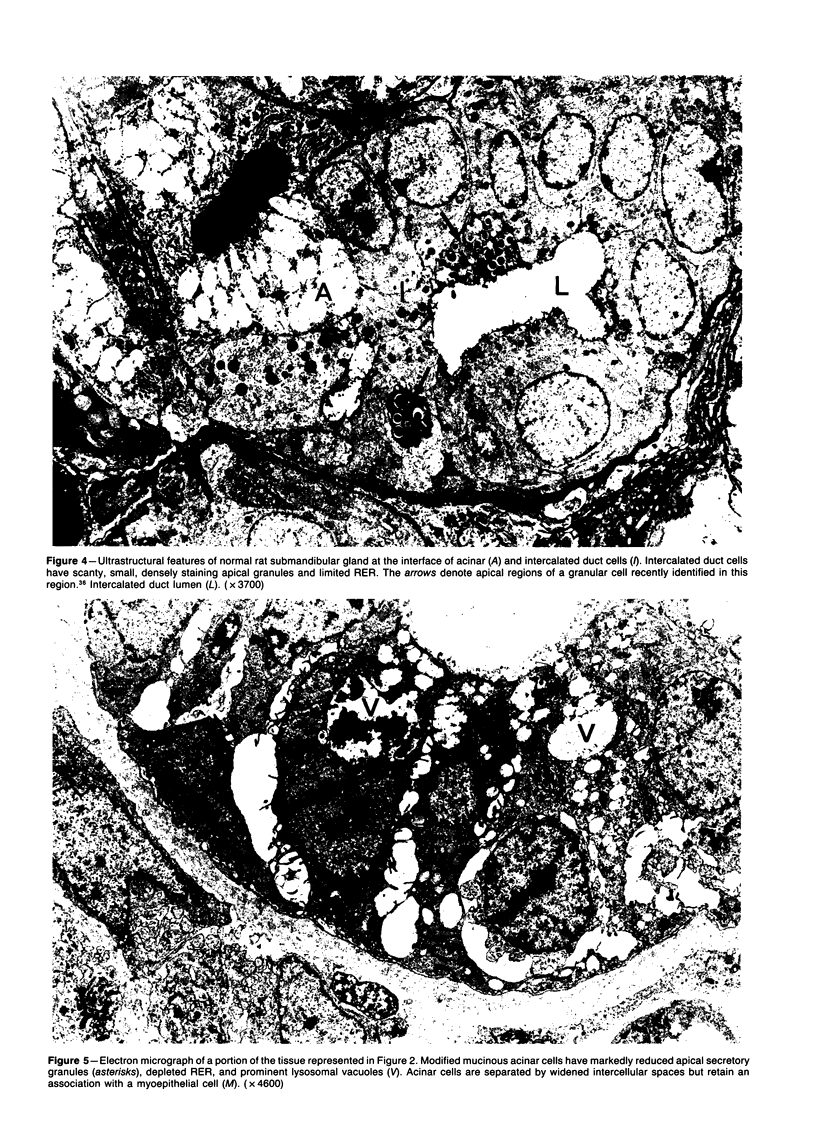


Images in this article
Selected References
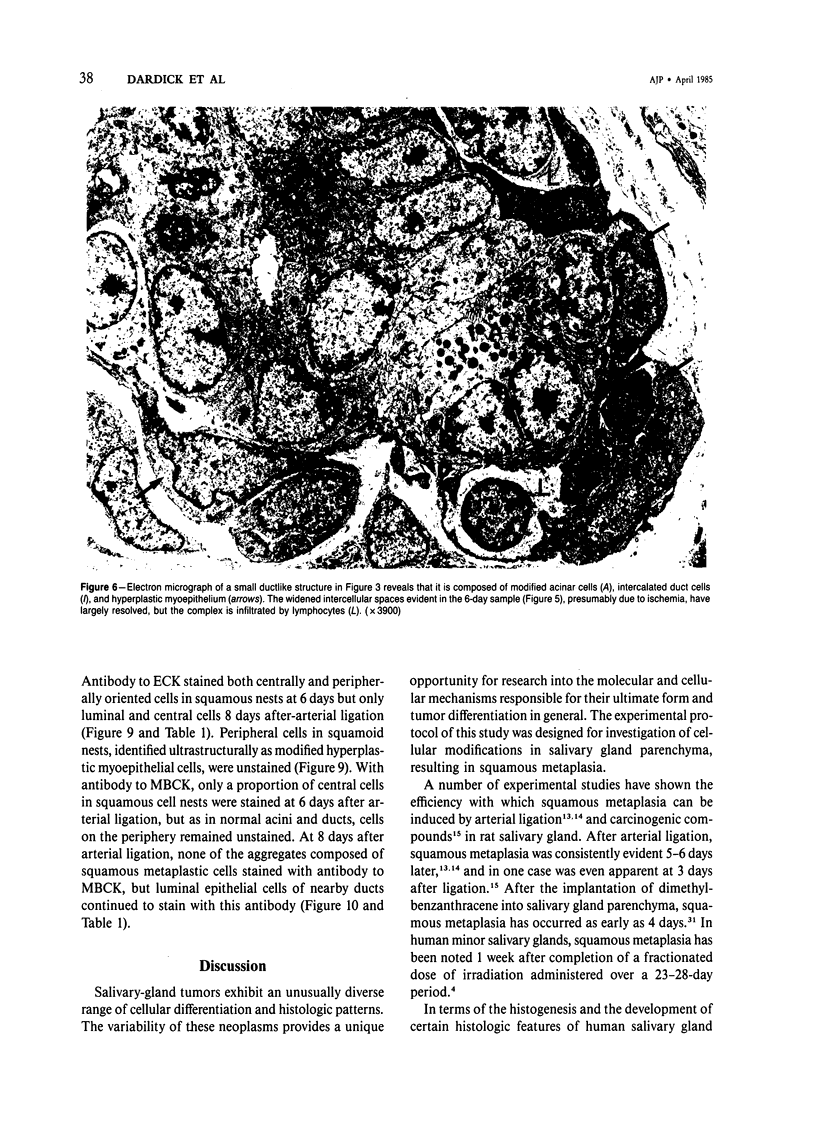
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. M., Melrose R. J., Howell F. V. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer. 1973 Jul;32(1):130–135. doi: 10.1002/1097-0142(197307)32:1<130::aid-cncr2820320118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Asch B. B., Burstein N. A., Vidrich A., Sun T. T. Identification of mouse mammary epithelial cells by immunofluorescence with rabbit and guinea pig antikeratin antisera. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5643–5647. doi: 10.1073/pnas.78.9.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biol M. C., Martin A., Alallon W., Richard M., Louisot P. Regulation of a soluble intestinal glycoprotein: fucosyl-transferase by fatty acids in vitro. Int J Biochem. 1981;13(8):927–933. doi: 10.1016/0020-711x(81)90020-3. [DOI] [PubMed] [Google Scholar]
- Bockman D. E. Cells of origin of pancreatic cancer: experimental animal tumors related to human pancreas. Cancer. 1981 Mar 15;47(6 Suppl):1528–1534. doi: 10.1002/1097-0142(19810315)47:6+<1528::aid-cncr2820471415>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Caselitz J., Löning T., Staquet M. J., Seifert G., Thivolet J. Immunocytochemical demonstration of filamentous structures in the parotid gland. Occurrence of keratin and actin in normal and tumoral parotid gland with special respect to the myoepithelial cells. J Cancer Res Clin Oncol. 1981;100(1):59–68. doi: 10.1007/BF00405902. [DOI] [PubMed] [Google Scholar]
- Daley T. D., Dardick I. An unusual parotid tumor with histogenetic implications for salivary gland neoplasms. Oral Surg Oral Med Oral Pathol. 1983 Apr;55(4):374–381. doi: 10.1016/0030-4220(83)90192-5. [DOI] [PubMed] [Google Scholar]
- Dardick I., Daya D., Hardie J., van Nostrand A. W. Mucoepidermoid carcinoma: ultrastructural and histogenetic aspects. J Oral Pathol. 1984 Aug;13(4):342–358. doi: 10.1111/j.1600-0714.1984.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Dardick I., Van Nostrand A. W., Jeans M. T., Rippstein P., Edwards V. Pleomorphic adenoma, II: Ultrastructural organization of "stromal" regions. Hum Pathol. 1983 Sep;14(9):798–809. doi: 10.1016/s0046-8177(83)80302-5. [DOI] [PubMed] [Google Scholar]
- Dardick I., van Nostrand A. W., Jeans M. T., Rippstein P., Edwards V. Pleomorphic adenoma, I: Ultrastructural organization of "epithelial" regions. Hum Pathol. 1983 Sep;14(9):780–797. doi: 10.1016/s0046-8177(83)80301-3. [DOI] [PubMed] [Google Scholar]
- Dardick I., van Nostrand A. W., Phillips M. J. Histogenesis of salivary gland pleomorphic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum Pathol. 1982 Jan;13(1):62–75. doi: 10.1016/s0046-8177(82)80140-8. [DOI] [PubMed] [Google Scholar]
- Donath K. Speicheldrüseninfarkt (Necrotizing Sialometaplasia) durch intraglanduläre Kreislaufstörung der Rattenparotis. Experimentelle Studie. Dtsch Zahnarztl Z. 1980 Jan;35(1):66–69. [PubMed] [Google Scholar]
- Englander A., Cataldo E. Experimental carcinogenesis in duct-artery ligated rat submandibular gland. J Dent Res. 1976 Mar-Apr;55(2):229–234. doi: 10.1177/00220345760550021101. [DOI] [PubMed] [Google Scholar]
- Eversole L. R. Histogenic classification of salivary tumors. Arch Pathol. 1971 Dec;92(6):433–443. [PubMed] [Google Scholar]
- FRIEDMAN M., HALL J. W. Radiation-induced squamous-cell metaplasia and hyperplasia of the normal mucous glands of the oral cavity. Radiology. 1950 Dec;55(6):848–851. doi: 10.1148/55.6.848. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H. J., Hanna W., Yeger H., Baumal R. Immunohistochemical localization of prekeratin filaments in benign and malignant cells in effusions. Comparison with intermediate filament distribution by electron microscopy. Am J Pathol. 1982 Nov;109(2):206–214. [PMC free article] [PubMed] [Google Scholar]
- Kahn H. J., Huang S. N., Hanna W. M., Baumal R., Phillips M. J. Immunohistochemical localization of epidermal and Mallory body cytokeratin in undifferentiated epithelial tumors. Comparison with ultrastructural features. Am J Clin Pathol. 1984 Feb;81(2):184–191. doi: 10.1093/ajcp/81.2.184. [DOI] [PubMed] [Google Scholar]
- Kimoff R. J., Huang S. Immunocytochemical and immunoelectron microscopic studies on Mallory bodies. Lab Invest. 1981 Dec;45(6):491–503. [PubMed] [Google Scholar]
- Krepler R., Denk H., Weirich E., Schmid E., Franke W. W. Keratin-like proteins in normal and neoplastic cells of human and rat mammary gland as revealed by immunofluorescence microscopy. Differentiation. 1981;20(3):242–252. doi: 10.1111/j.1432-0436.1981.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Krüger R., Dietrich H. G. Der Speicheldrüseninfarkt (Necrotizing Sialometaplasia)--Differentialdiagnose und Pathogenese. Pathologe. 1982 Nov;3(6):342–347. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Regezi J. A., Batsakis J. G. Histogenesis of salivary gland neoplasms. Otolaryngol Clin North Am. 1977 Jun;10(2):297–307. [PubMed] [Google Scholar]
- STANDISH S. M. Early histologic changes in induced tumors of the submaxillary salivary glands of the rat. Am J Pathol. 1957 Jul-Aug;33(4):671–689. [PMC free article] [PubMed] [Google Scholar]
- STANDISH S. M., SHAFER W. G. Serial histologic effects of rat submaxillary and sublingual salivary gland duct and blood vessel ligation. J Dent Res. 1957 Dec;36(6):866–879. doi: 10.1177/00220345570360060801. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]