Abstract
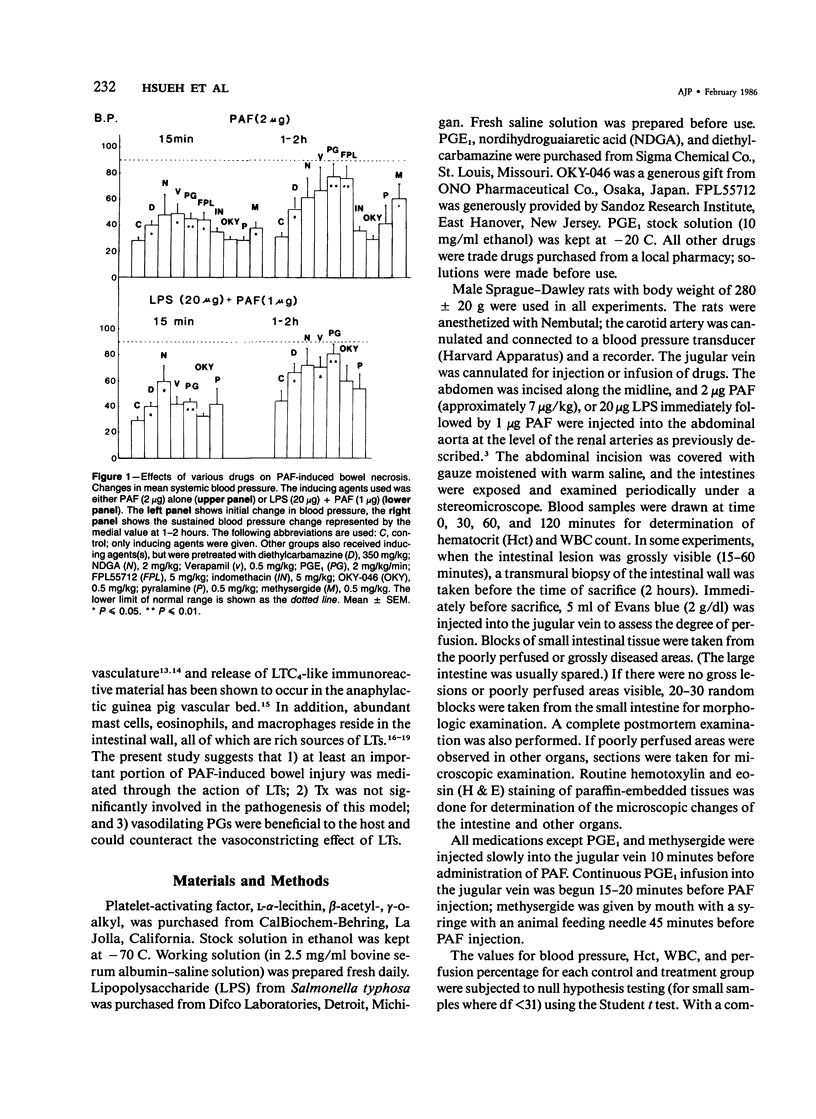
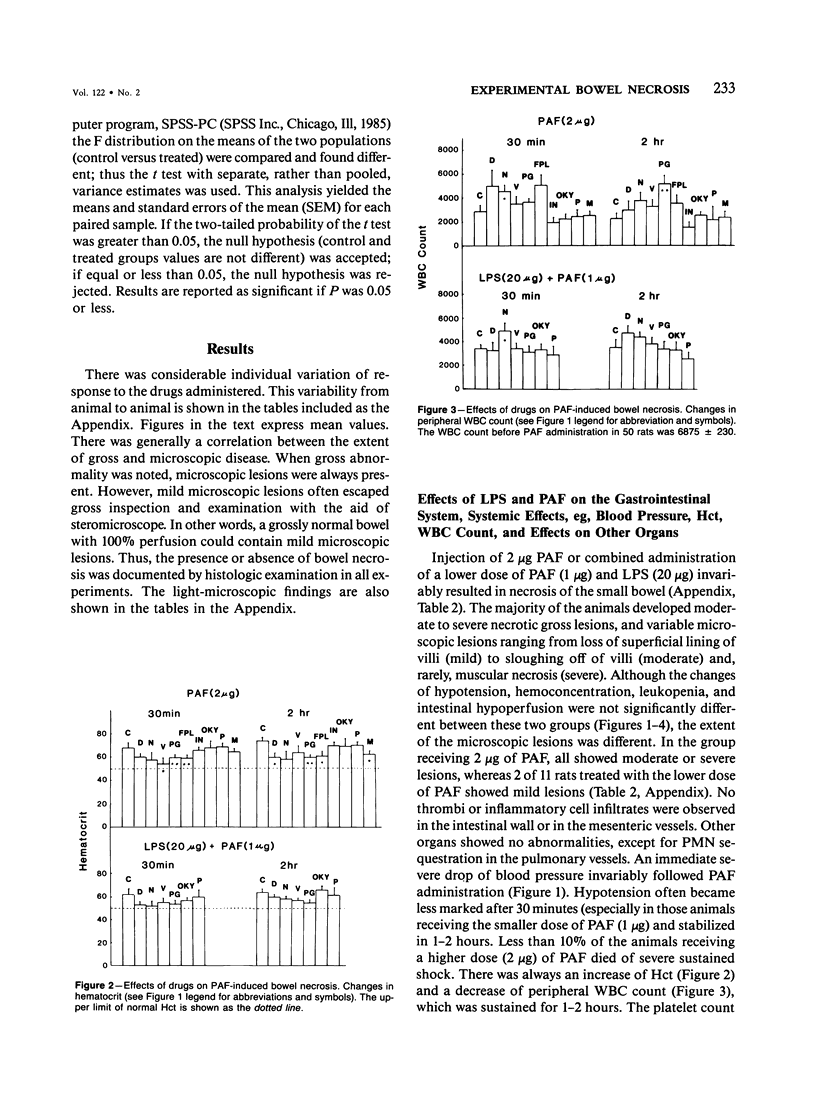
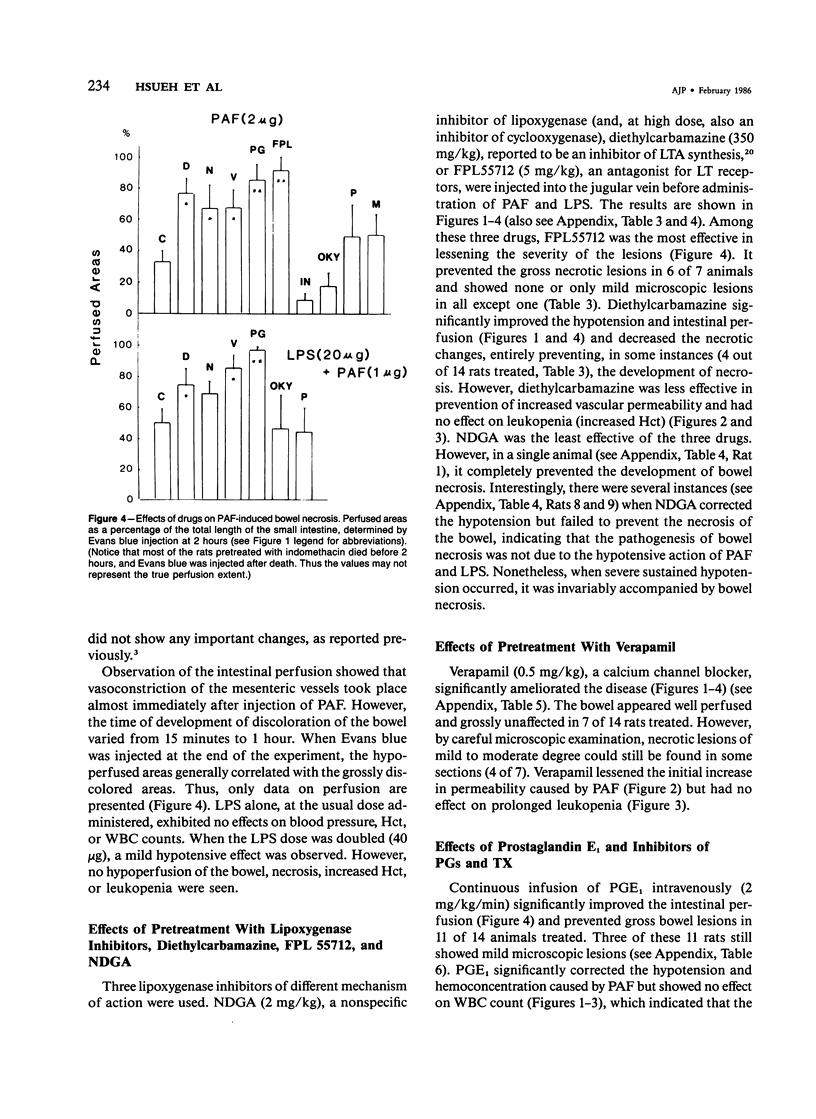
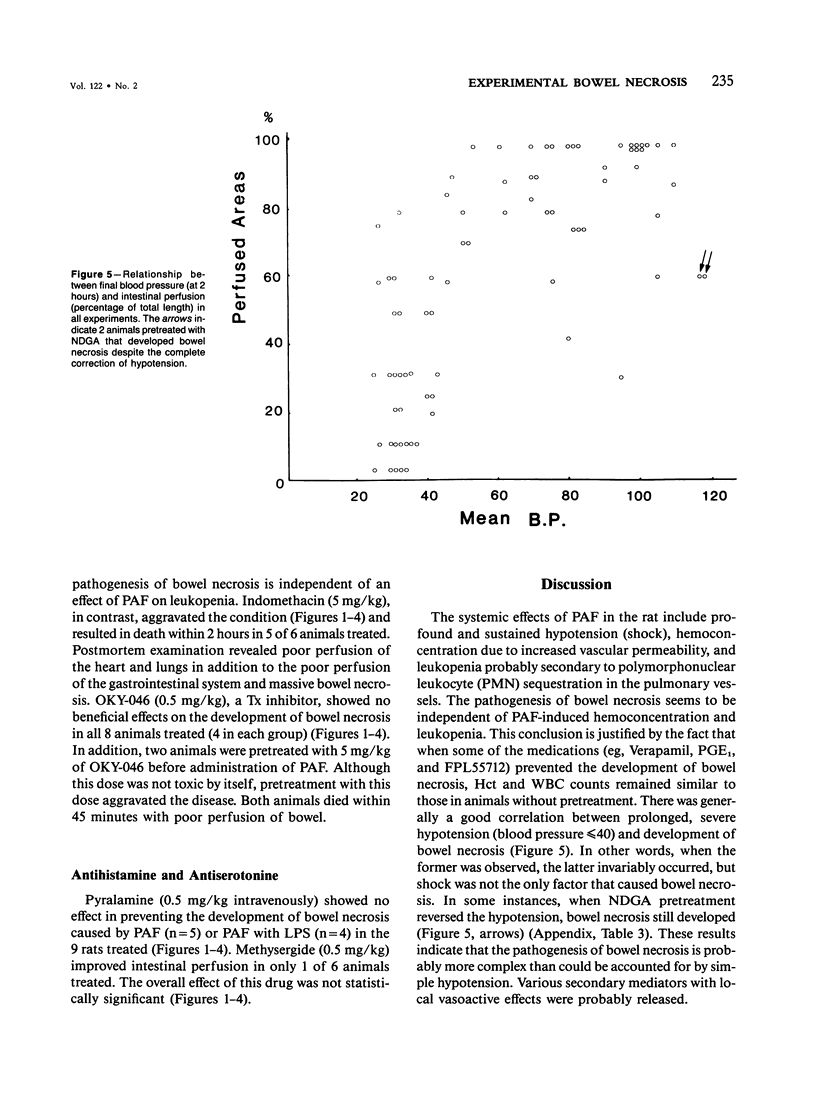
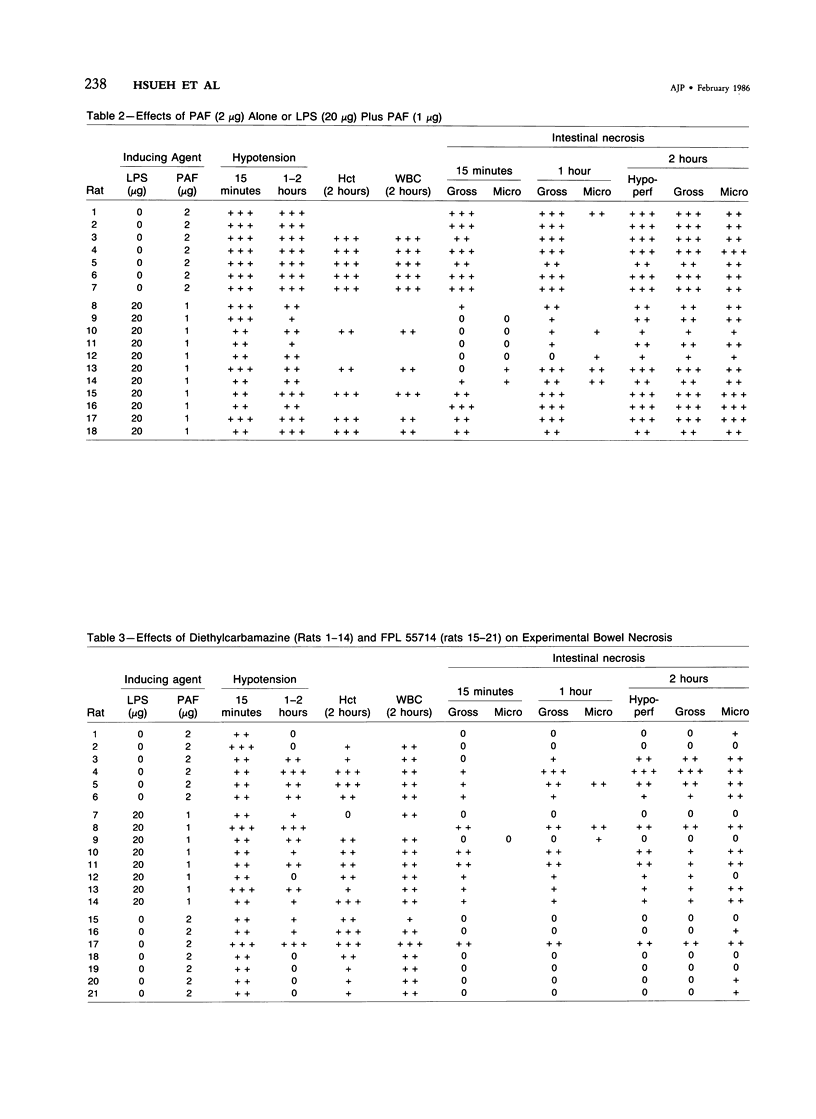
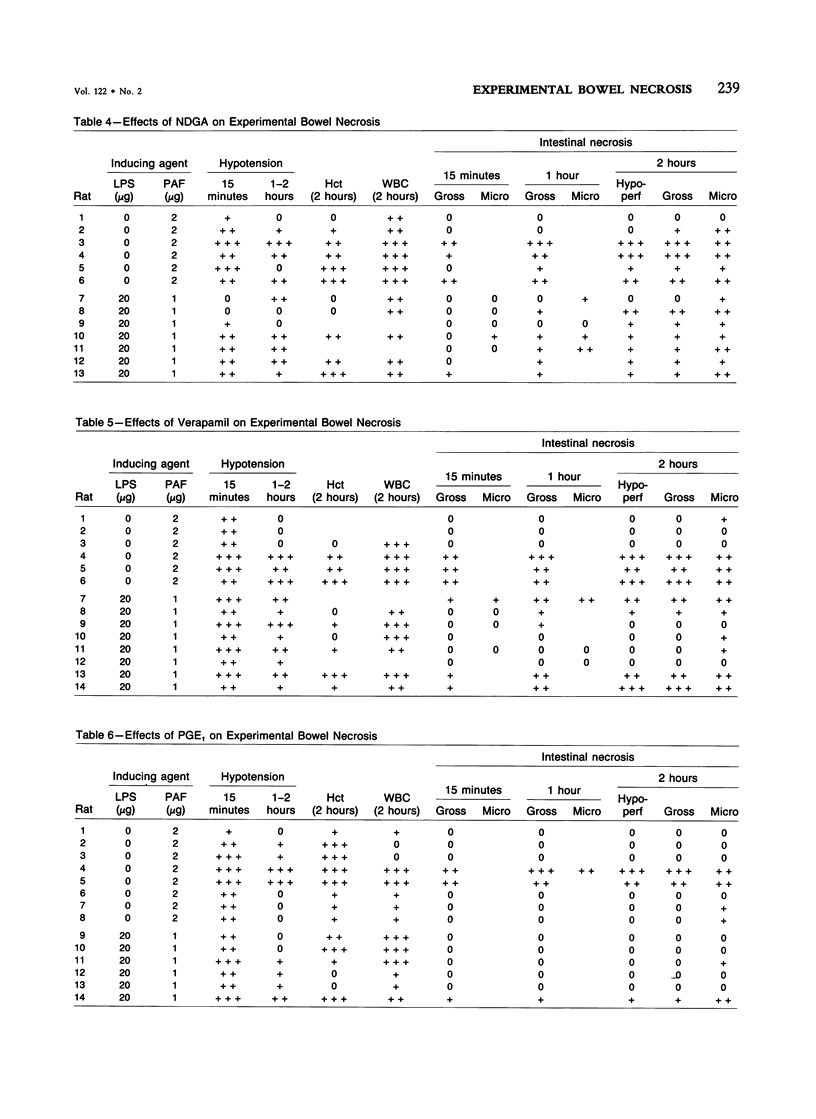
The authors have previously reported a model of ischemic bowel necrosis produced in the rat by synthetic platelet-activating factor (PAF) or a combination of PAF and bacterial endotoxin. Because rat platelets are refractory to PAF and thromboemboli were not found in the mesenteric or intestinal microvasculature, they suspected that secondary mediators were involved in the pathogenesis of bowel necrosis. They have found the following lipoxygenase products of arachidonic acid, especially leukotrienes (LT), probably played an important role in the pathogenesis of bowel necrosis, because diethylcarbamazine (an inhibitor for LTA synthesis) and FPL55712 (LT antagonist) ameliorated, and at times completely prevented, the lesions. NDGA (a nonspecific lipoxygenase inhibitor) was less effective, probably because of its additional effect on cyclooxygenase inhibition. Verapamil, a calcium channel blocker, ameliorated the disease. Thromboxane A2, a potent vasoconstrictor, was probably not responsible for the ischemia of the gastrointestinal tract. This is suggested by the ineffectiveness of OKY-046 in preventing bowel necrosis. Prostaglandin (PG) E1 infusion often prevented the bowel necrosis, which suggested beneficial effects of vasodilating PGs, probably released as a defense mechanisms. Indomethacin aggravated the disease, probably by inhibiting PG release and shifting the metabolic pathway toward the lipoxygenase pathway. Antihistamine and antiserotonin had no effect, which suggested that these mediators were not involved in the pathogenesis of bowel necrosis. Shock produced by PAF was probably not the only cause of bowel necrosis, because reversal of the hypotension did not always prevent the development of bowel necrosis. Hemoconcentration (increased vasopermeability) and leukopenia induced by PAF did not correlate with the development or severity of bowel necrosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Cashore W. J., Peter G., Lauermann M., Stonestreet B. S., Oh W. Clostridia colonization and clostridial toxin in neonatal necrotizing enterocolitis. J Pediatr. 1981 Feb;98(2):308–311. doi: 10.1016/s0022-3476(81)80667-1. [DOI] [PubMed] [Google Scholar]
- Chapnick B. M. Divergent influences of leukotrienes C4, D4, and E4 on mesenteric and renal blood flow. Am J Physiol. 1984 Apr;246(4 Pt 2):H518–H524. doi: 10.1152/ajpheart.1984.246.4.H518. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Walsh C. E., Thomas M. J., Wykle R. L., DeChatelet L. R., Waite B. M. Platelet activating factor. Stimulation of the lipoxygenase pathway in polymorphonuclear leukocytes by 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. J Biol Chem. 1982 May 25;257(10):5402–5407. [PubMed] [Google Scholar]
- Dembinska-Kieć A., Simmet T., Peskar B. A. Release and vasoconstrictor effect of leukotriene C-like immunoreactive material in the anaphylactic guinea-pig mesenteric vascular bed. Eur J Pharmacol. 1984 Jun 1;101(3-4):259–262. doi: 10.1016/0014-2999(84)90166-3. [DOI] [PubMed] [Google Scholar]
- Feigen L. P. Differential effects of leukotrienes C4, D4 and E4 in the canine renal and mesenteric vascular beds. J Pharmacol Exp Ther. 1983 Jun;225(3):682–687. [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983 Jul;112(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Kliegman R. M. Neonatal necrotizing enterocolitis: implications for an infectious disease. Pediatr Clin North Am. 1979 May;26(2):327–344. doi: 10.1016/s0031-3955(16)33709-9. [DOI] [PubMed] [Google Scholar]
- Lawrence G., Walker P. D. Pathogenesis of enteritis necroticans in Papula New Guinea. Lancet. 1976 Jan 17;1(7951):125–126. doi: 10.1016/s0140-6736(76)93160-3. [DOI] [PubMed] [Google Scholar]
- LeDuc L. E., Needleman P. Regional localization of prostacyclin and thromboxane synthesis in dog stomach and intestinal tract. J Pharmacol Exp Ther. 1979 Oct;211(1):181–188. [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Schleimer R. P., Peters S. P., Schulman E. S., Adams G. K., 3rd, Newball H. H., Lichtenstein L. M. Generation of leukotrienes by purified human lung mast cells. J Clin Invest. 1982 Oct;70(4):747–751. doi: 10.1172/JCI110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews W. R., Murphy R. C. Inhibition of leukotriene biosynthesis in mastocytoma cells by diethylcarbamazine. Biochem Pharmacol. 1982 Jun 1;31(11):2129–2132. doi: 10.1016/0006-2952(82)90435-x. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Benveniste J. Platelet-activating factor (PAF-acether) and macrophages. II. Phagocytosis-associated release of PAF-acether from rat peritoneal macrophages. Cell Immunol. 1981 Jan 15;57(2):281–292. doi: 10.1016/0008-8749(81)90087-3. [DOI] [PubMed] [Google Scholar]
- Peskar B. M., Günter B., Peskar B. A. Prostaglandins and prostaglandin metabolites in human gastric juice. Prostaglandins. 1980 Aug;20(2):419–427. doi: 10.1016/s0090-6980(80)80059-1. [DOI] [PubMed] [Google Scholar]
- Peskar B. M., Weiler H., Kröner E. E., Peskar B. A. Release of prostaglandins by small intestinal tissue of man and rat in vitro and the effect of endotoxin in the rat in vivo. Prostaglandins. 1981;21 (Suppl):9–14. doi: 10.1016/0090-6980(81)90111-8. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., McManus L. M., Halonen M., Hanahan D. J. Acetyl glyceryl ether phosphorylcholine: platelet-activating factor. Int Arch Allergy Appl Immunol. 1981;66 (Suppl 1):127–136. doi: 10.1159/000232884. [DOI] [PubMed] [Google Scholar]
- Razin E., Mencia-Huerta J. M., Lewis R. A., Corey E. J., Austen K. F. Generation of leukotriene C4 from a subclass of mast cells differentiated in vitro from mouse bone marrow. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4665–4667. doi: 10.1073/pnas.79.15.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Cohn Z. A., Blackburn P., Manning J. M. Mouse peritoneal macrophages release leukotriene C in response to a phagocytic stimulus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4928–4932. doi: 10.1073/pnas.77.8.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. S., Warhurst G., Turnberg L. A. Synthesis and degradation of prostaglandin E2 in the epithelial and sub-epithelial layers of the rat intestine. Biochim Biophys Acta. 1982 Dec 13;713(3):684–687. doi: 10.1016/0005-2760(82)90331-9. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Worthen S., Reeves J. T., Henson P. M., Murphy R. C. Nonimmunological production of leukotrienes induced by platelet-activating factor. Science. 1982 Oct 15;218(4569):286–289. doi: 10.1126/science.7123233. [DOI] [PubMed] [Google Scholar]
- Walker R., Wilson K. A. Gastroenterology, prostaglandins, bradykinin, and rat ileum. Adv Prostaglandin Thromboxane Res. 1980;8:1573–1575. [PubMed] [Google Scholar]
- Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]


