Abstract
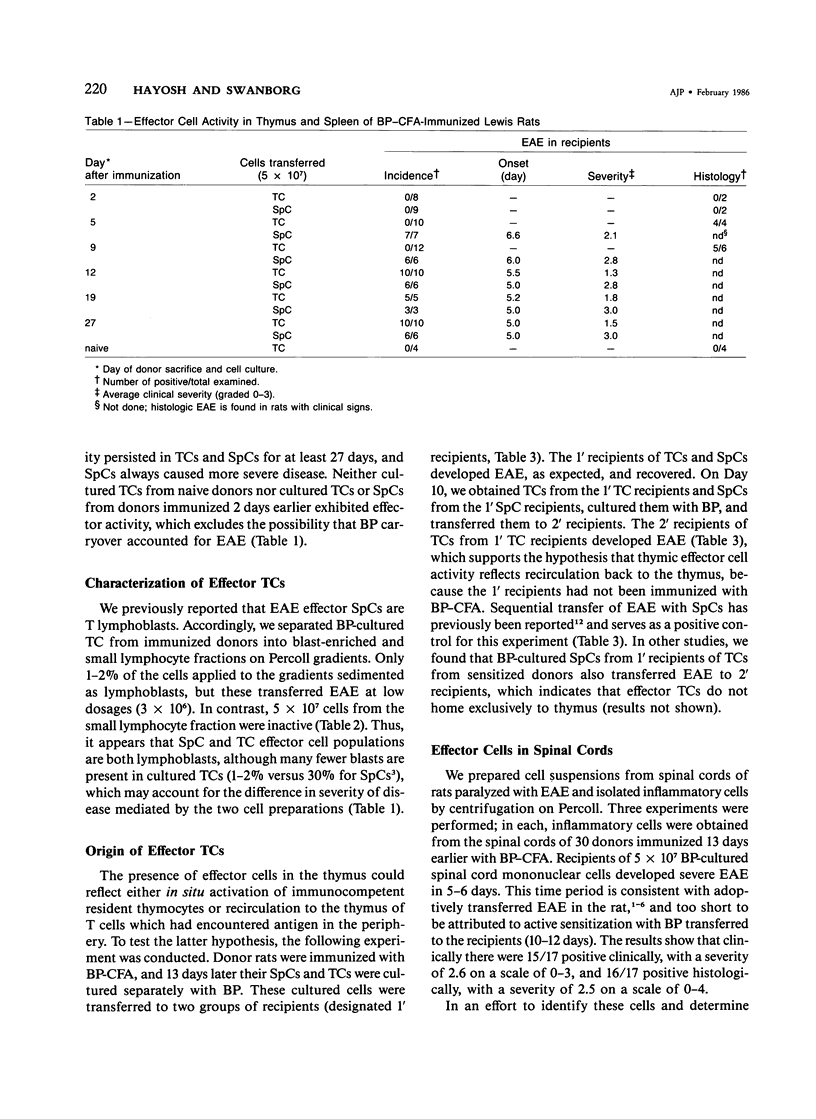
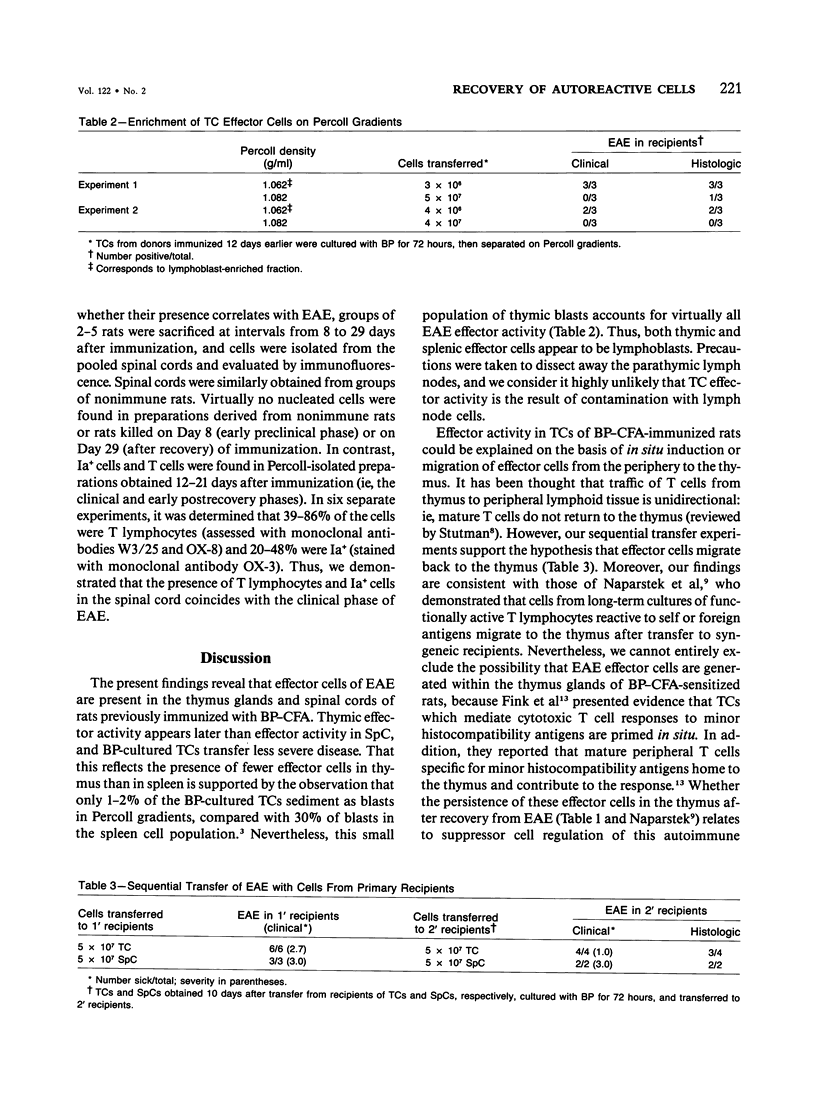
Effector cells mediating experimental allergic encephalomyelitis (EAE) were recovered from the thymus glands and spinal cords of Lewis rats immunized with myelin basic protein (BP) and complete Freund's adjuvant (CFA), as demonstrated by adoptive transfer to syngeneic recipients following in vitro activation with BP. The thymic effector cells (TCs) were lymphoblasts. Sequential transfer studies suggested that the effector TCs probably recirculated back to the thymus from the periphery. The transfer of EAE with cells derived from the spinal cords of paralyzed donors indicates that disease-inducing effector cells are present in the target organ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J., Rosenzweig A., Zweiman B., Moskovitz A., Lisak R. Recovery of myelin basic protein reactive T cells from spinal cords of Lewis rats with autoimmune encephalomyelitis. J Immunol. 1984 Jun;132(6):2690–2692. [PubMed] [Google Scholar]
- Driscoll B. F., Kies M. W., Alvord E. C., Jr Transfer of experimental allergic encephalomyelitis with guinea pig peritoneal exudate cells. Science. 1979 Feb 9;203(4380):547–548. doi: 10.1126/science.83676. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J., Weissman I. L. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J Exp Med. 1984 Feb 1;159(2):436–451. doi: 10.1084/jem.159.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayosh N. S., Simon L. L., Swanborg R. H. Autoimmune effector cells. VI. Transfer of experimental allergic encephalomyelitis with spleen cells activated in mixed lymphocyte cultures. J Immunol. 1984 Oct;133(4):1943–1945. [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K., Kimura H., Wilson D. B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983 Dec;131(6):2805–2809. [PubMed] [Google Scholar]
- Hinrichs D. J., Roberts C. M., Waxman F. J. Regulation of paralytic experimental allergic encephalomyelitis in rats: susceptibility to active and passive disease reinduction. J Immunol. 1981 May;126(5):1857–1862. [PubMed] [Google Scholar]
- Holda J. H., Silberg D., Swanborg R. H. Autoimmune effector cells. IV. Induction of experimental allergic encephalomyelitis in Lewis rats without adjuvant. J Immunol. 1983 Feb;130(2):732–734. [PubMed] [Google Scholar]
- Holda J. H., Swanborg R. H. Autoimmune effector cells. II. Transfer of experimental allergic encephalomyelitis with a subset of T lymphocytes. Eur J Immunol. 1982 May;12(5):453–455. doi: 10.1002/eji.1830120519. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Holoshitz J., Eisenstein S., Reshef T., Rappaport S., Chemke J., Ben-Nun A., Cohen I. R. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982 Nov 18;300(5889):262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- Ovadia H., Paterson P. Y. Cellular transfer of experimental allergic encephalomyelitis in Lewis rats: effects of different sensitization regimens on in vitro reactivity of donor spleen cells to concanavalin A. Cell Immunol. 1981 Nov 15;65(1):66–74. doi: 10.1016/0008-8749(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., McFarlin D. E. Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol. 1977 Sep;119(3):1134–1137. [PubMed] [Google Scholar]
- Richert J. R., Driscoll B. F., Kies M. W., Alvord E. C., Jr Adoptive transfer of experimental allergic encephalomyelitis: incubation of rat spleen cells with specific antigen. J Immunol. 1979 Feb;122(2):494–496. [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. I. Quantitative analysis of inflammatory cells in situ. J Immunol. 1984 May;132(5):2393–2401. [PubMed] [Google Scholar]
- Sriram S., Solomon D., Rouse R. V., Steinman L. Identification of T cell subsets and B lymphocytes in mouse brain experimental allergic encephalitis lesions. J Immunol. 1982 Oct;129(4):1649–1651. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Swanborg R. H. Autoimmune effector cells. V.A monoclonal antibody specific for rat helper T lymphocytes inhibits adoptive transfer of autoimmune encephalomyelitis. J Immunol. 1983 Apr;130(4):1503–1505. [PubMed] [Google Scholar]
- Traugott U., Raine C. S., McFarlin D. E. Acute experimental allergic encephalomyelitis in the mouse: immunopathology of the developing lesion. Cell Immunol. 1985 Mar;91(1):240–254. doi: 10.1016/0008-8749(85)90047-4. [DOI] [PubMed] [Google Scholar]
- Traugott U., Shevach E., Chiba J., Stone H. J., Raine C. S. Autoimmune encephalomyelitis: simultaneous identification of T and B cells in the target organ. Science. 1981 Dec 11;214(4526):1251–1253. doi: 10.1126/science.7029715. [DOI] [PubMed] [Google Scholar]
- Welch A. M., Holda J. H., Swanborg R. H. Regulation of experimental allergic encephalomyelitis. II. Appearance of suppressor cells during the remission phase of the disease. J Immunol. 1980 Jul;125(1):186–189. [PubMed] [Google Scholar]
- Werdelin O., McCluskey R. T. The nature and the specificity of mononuclear cells in experimental autoimmune inflammations and the mechanisms leading to their accumulation. J Exp Med. 1971 Jun 1;133(6):1242–1263. doi: 10.1084/jem.133.6.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. M., Bloom B. R. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med. 1975 Feb 1;141(2):346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]


