Abstract
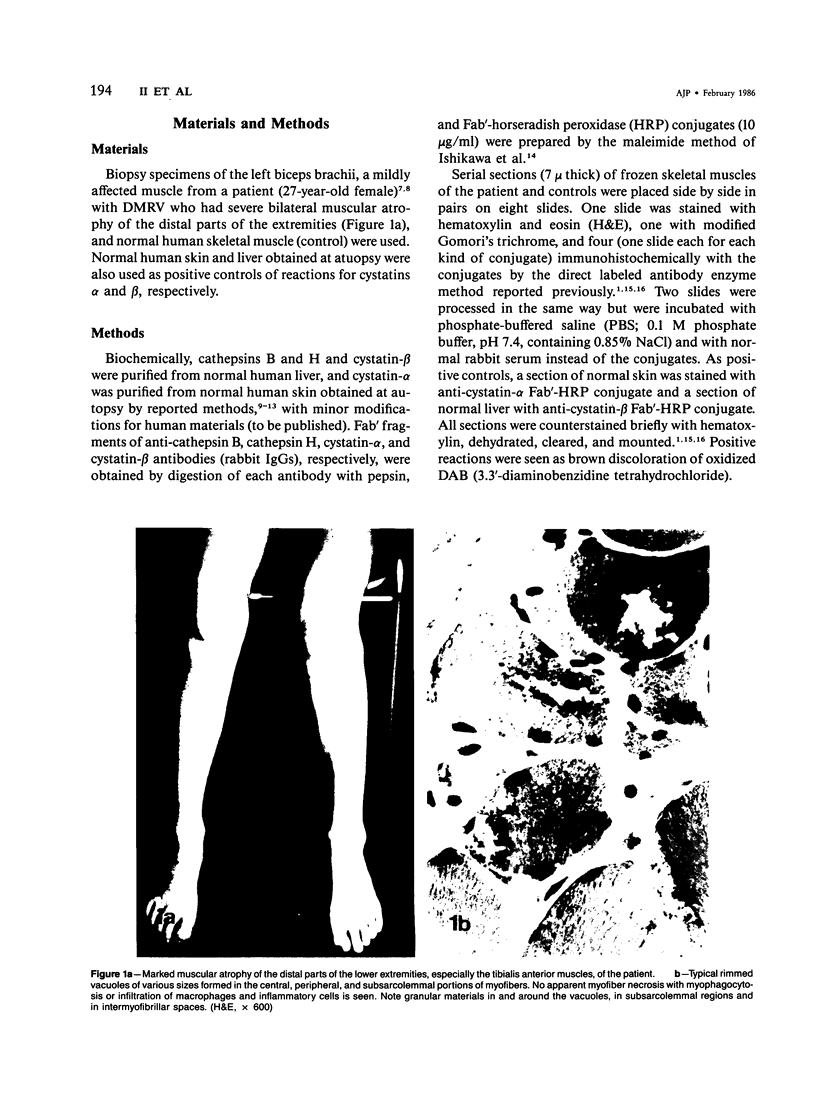
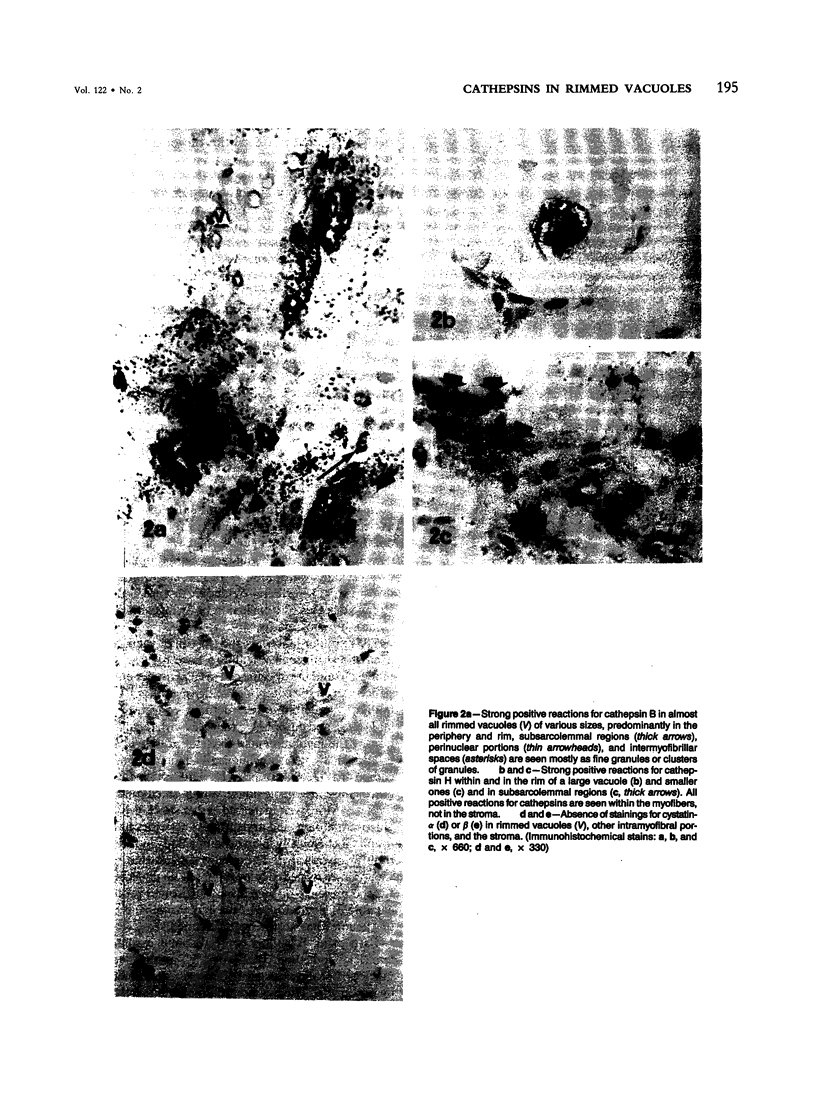
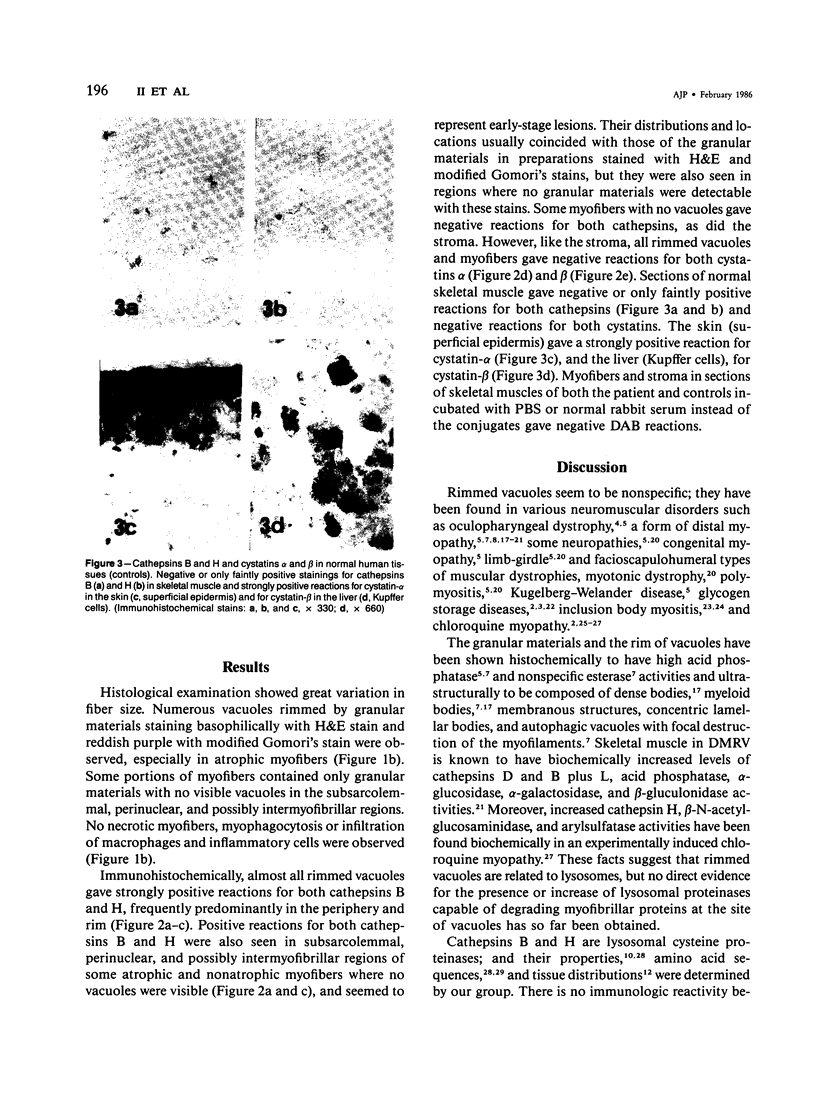
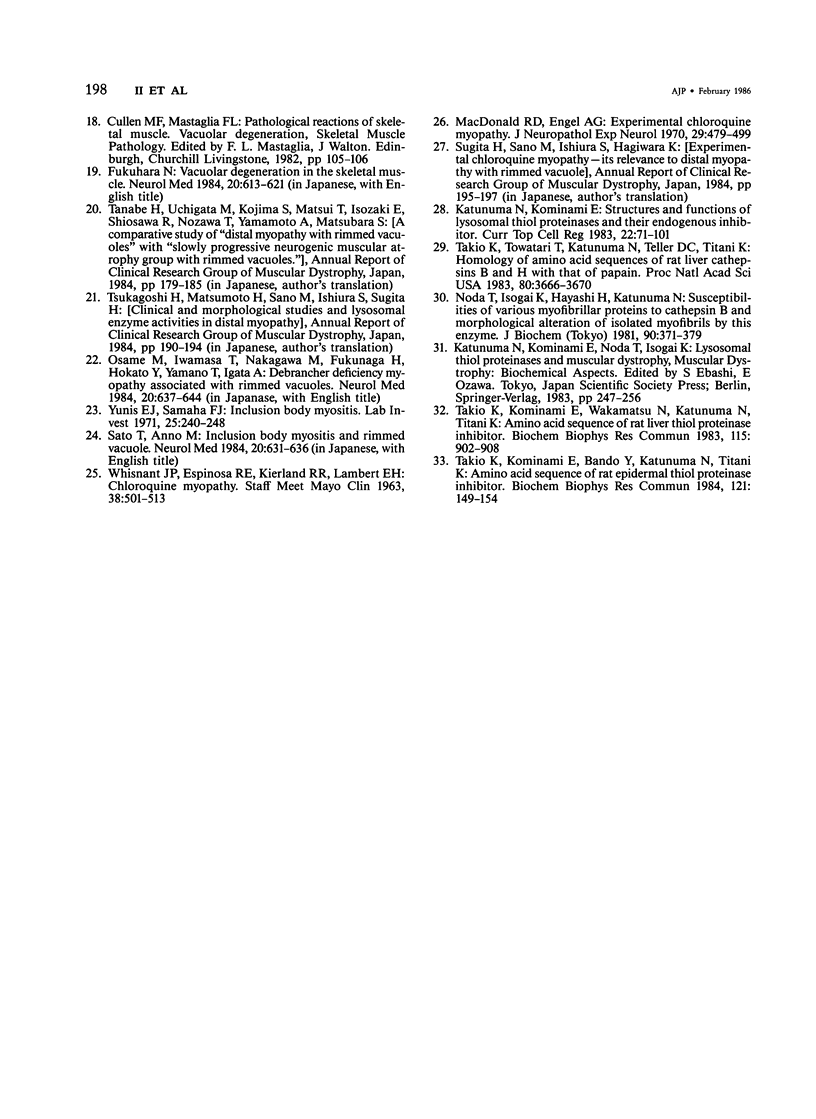
Skeletal muscle obtained from a patient with distal myopathy with rimmed vacuole formation (DMRV) was examined by a new direct labeled antibody enzyme method of immunohistochemistry. Abnormal increases of cathepsins B and H, capable of degrading the myofibrillar proteins, were demonstrated to be localized at the site of vacuoles and other intramyofibral portions and not to be associated with concomitant increases of their endogenous inhibitors. Autodigestion by these intramyofibral lysosomal proteinases may be of major importance in focal destruction of myofibers and formation of vacuoles, because no myofiber necrosis with invasion of macrophages, overt myositis, and neuropathy was seen in the muscle. These findings provide the first reasonable explanation of myofibral breakdown and atrophy in DMRV, and should be helpful in further studies on the mechanism of myofibral breakdown in various vacuolar myopathies and other myopathies of so far unknown pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukuhara N., Kumamoto T., Tsubaki T. Rimmed vacuoles. Acta Neuropathol. 1980;51(3):229–235. doi: 10.1007/BF00687390. [DOI] [PubMed] [Google Scholar]
- Ii K., Hizawa K., Kominami E., Bando Y., Katunuma N. Different immunolocalizations of cathepsins B, H, and L in the liver. J Histochem Cytochem. 1985 Nov;33(11):1173–1175. doi: 10.1177/33.11.4056381. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Imagawa M., Hashida S., Yoshitake S., Hamaguchi Y., Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4(3):209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Structures and functions of lysosomal thiol proteinases and their endogenous inhibitor. Curr Top Cell Regul. 1983;22:71–101. doi: 10.1016/b978-0-12-152822-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Hanson H. Cathepsin H: an endoaminopeptidase from rat liver lysosomes. Acta Biol Med Ger. 1977;36(2):185–199. [PubMed] [Google Scholar]
- Kominami E., Bando Y., Wakamatsu N., Katunuma N. Different tissue distributions of two types of thiol proteinase inhibitors from rat liver and epidermis. J Biochem. 1984 Nov;96(5):1437–1442. doi: 10.1093/oxfordjournals.jbchem.a134972. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Bando Y., Katunuma N. Distribution of cathepsins B and H in rat tissues and peripheral blood cells. J Biochem. 1985 Jul;98(1):87–93. doi: 10.1093/oxfordjournals.jbchem.a135277. [DOI] [PubMed] [Google Scholar]
- Kominami E., Wakamatsu N., Katunuma N. Purification and characterization of thiol proteinase inhibitor from rat liver. J Biol Chem. 1982 Dec 25;257(24):14648–14652. [PubMed] [Google Scholar]
- Macdonald R. D., Engel A. G. Experimental chloroquine myopathy. J Neuropathol Exp Neurol. 1970 Jul;29(3):479–499. doi: 10.1097/00005072-197007000-00010. [DOI] [PubMed] [Google Scholar]
- Markesbery W. R., Griggs R. C., Herr B. Distal myopathy: electron microscopic and histochemical studies. Neurology. 1977 Aug;27(8):727–735. doi: 10.1212/wnl.27.8.727. [DOI] [PubMed] [Google Scholar]
- Noda T., Isogai K., Hayashi H., Katunuma N. Susceptibilities of various myofibrillar proteins to cathepsin B and morphological alteration of isolated myofibrils by this enzyme. J Biochem. 1981 Aug;90(2):371–379. doi: 10.1093/oxfordjournals.jbchem.a133483. [DOI] [PubMed] [Google Scholar]
- Nonaka I., Sunohara N., Ishiura S., Satoyoshi E. Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci. 1981 Jul;51(1):141–155. doi: 10.1016/0022-510x(81)90067-8. [DOI] [PubMed] [Google Scholar]
- Nonaka I., Sunohara N., Satoyoshi E., Terasawa K., Yonemoto K. Autosomal recessive distal muscular dystrophy: a comparative study with distal myopathy with rimmed vacuole formation. Ann Neurol. 1985 Jan;17(1):51–59. doi: 10.1002/ana.410170113. [DOI] [PubMed] [Google Scholar]
- Takio K., Kominami E., Bando Y., Katunuma N., Titani K. Amino acid sequence of rat epidermal thiol proteinase inhibitor. Biochem Biophys Res Commun. 1984 May 31;121(1):149–154. doi: 10.1016/0006-291x(84)90699-5. [DOI] [PubMed] [Google Scholar]
- Takio K., Kominami E., Wakamatsu N., Katunuma N., Titani K. Amino acid sequence of rat liver thiol proteinase inhibitor. Biochem Biophys Res Commun. 1983 Sep 30;115(3):902–908. doi: 10.1016/s0006-291x(83)80020-5. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari T., Kawabata Y., Katunuma N. Crystallization and properties of cathepsin B from rat liver. Eur J Biochem. 1979 Dec;102(1):279–289. doi: 10.1111/j.1432-1033.1979.tb06290.x. [DOI] [PubMed] [Google Scholar]
- WHISNANT J. P., ESPINOSA R. E., KIERLAND R. R., LAMBERT E. H. CHLOROQUINE NEUROMYOPATHY. Proc Staff Meet Mayo Clin. 1963 Nov 6;38:501–513. [PubMed] [Google Scholar]
- Yunis E. J., Samaha F. J. Inclusion body myositis. Lab Invest. 1971 Sep;25(3):240–248. [PubMed] [Google Scholar]