Abstract
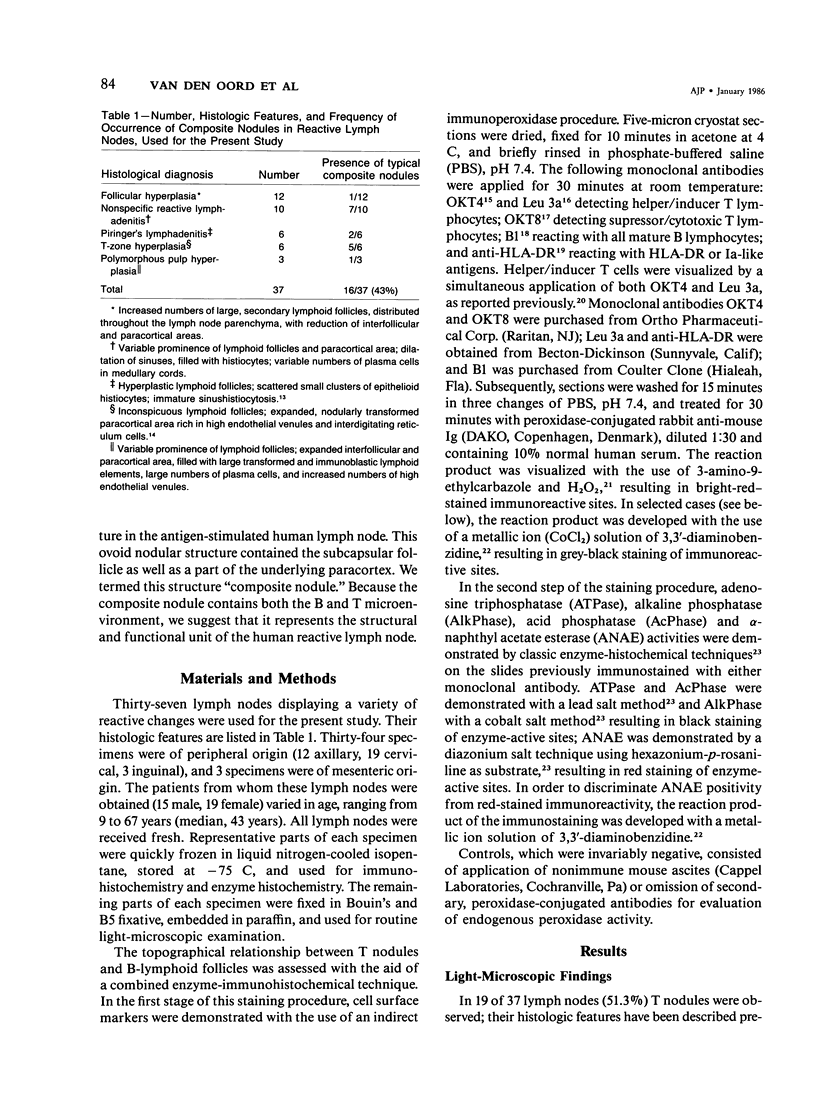
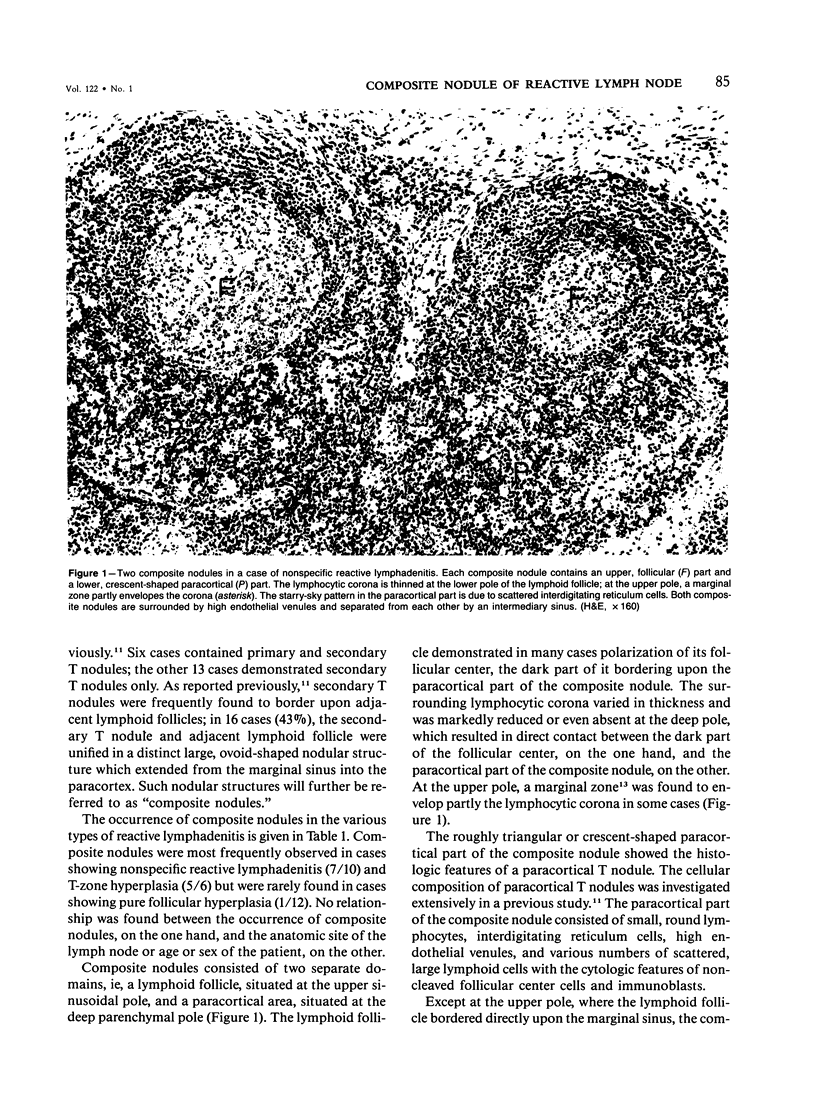
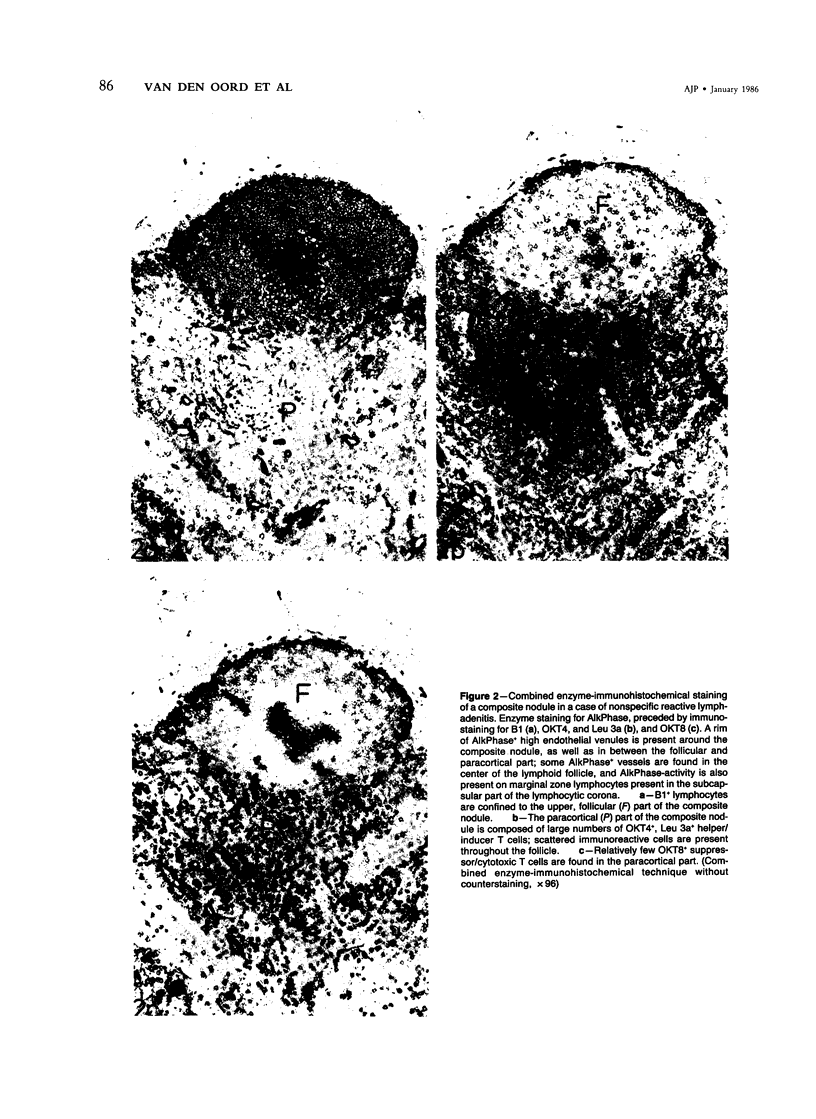
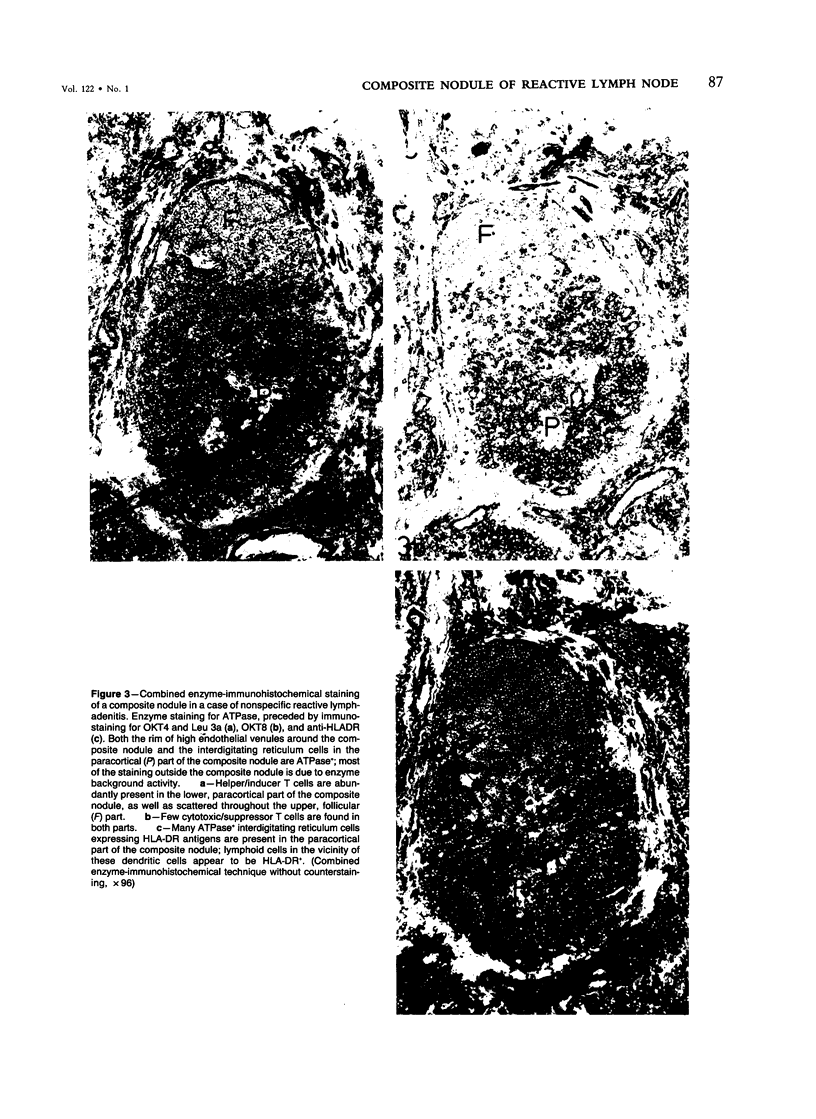
The relationship between T nodules and adjacent B-lymphoid follicles was investigated in 37 reactive lymph nodes by light microscopy and combined enzyme immunohistochemistry. In 16 cases (43%), T nodules and adjacent B-lymphoid follicles were unified in an ovoid, distinct nodular structure termed a "composite nodule." The composite nodule comprises two separate domains. The peripheral, subcapsular B domain contains all stationary and migratory elements of the B-lymphoid follicle, ie, B1+ B-cells, OKT4+, Leu 3a+ helper/inducer T cells, HLA-DR+ dendritic reticulum cells, and ANAE+, AcPhase+ tangible body macrophages and is surrounded by a B1+, HLA-DR+ lymphocytic corona displaying focal adenosine triphosphatase (ATPase) and alkaline phosphatase (AlkPhase) activity. The deep, paracortical T-domain contains all elements of the T nodule, ie, OKT4+, Leu3a+ helper/inducer T cells, high endothelial venules and HLA-DR+, ATPase+ interdigitating reticulum cells. The composite nodule is surrounded by a rim of ATPase+, AlkPhase+ high endothelial venules. Both domains are subject to changes in volume; thus, in follicular hyperplasia, the B domain enlarges at the cost of the T domain, and the reverse may occur in T-zone hyperplasia. Based on the striking resemblance between the composite nodule and the white pulp of the spleen, it is suggested that the composite nodule plays a major role in the triggering, helper-T-cell-dependent stimulation and subsequent maturation of antigen-responsive B cells into antibody-secreting plasma cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bélisle C., Sainte-Marie G. Topography of the deep cortex of the lymph nodes of various mammalian species. Anat Rec. 1981 Nov;201(3):553–561. doi: 10.1002/ar.1092010311. [DOI] [PubMed] [Google Scholar]
- Bélisle C., Sainte-Marie G. Tridimensional study of the deep cortex of the rat lymph node. I: Topography of the deep cortex. Anat Rec. 1981 Jan;199(1):45–59. doi: 10.1002/ar.1091990106. [DOI] [PubMed] [Google Scholar]
- Bélisle C., Sainte-Marie G. Tridimensional study of the deep cortex of the rat lymph node. III. Morphology of the deep cortex units. Anat Rec. 1981 Feb;199(2):213–226. doi: 10.1002/ar.1091990206. [DOI] [PubMed] [Google Scholar]
- Cottier H., Turk J., Sobin L. A proposal for a standardized system of reporting human lymph node morphology in relation to immunological function. Bull World Health Organ. 1972;47(3):375–417. [PMC free article] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Grogan T. M., Jolley C. S., Rangel C. S. Immunoarchitecture of the human spleen. Lymphology. 1983 Jun;16(2):72–82. [PubMed] [Google Scholar]
- Herman P. G., Yamamoto I., Mellins H. Z. Blood microcirculation in the lymph node during the primary immune response. J Exp Med. 1972 Oct 1;136(4):697–714. doi: 10.1084/jem.136.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Cossman J., Jaffe E. S. Lymphocyte subsets in normal human lymphoid tissues. Am J Clin Pathol. 1983 Jul;80(1):21–30. doi: 10.1093/ajcp/80.1.21. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 1. Identification with monoclonal antibodies in normal lymphoid tissues. Am J Pathol. 1984 Mar;114(3):387–395. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Inaba K., Witmer M. D., Steinman R. M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J Exp Med. 1984 Sep 1;160(3):858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H., Kunkl A., Dongworth D. W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Kojima M., Takahashi K., Sue A., Imai Y. Study on the function and structure of blood vessels of the secondary nodules in lymph node. Acta Pathol Jpn. 1971 Aug;21(3):369–386. doi: 10.1111/j.1440-1827.1971.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Crosby W. H. Histochemical characterization of cellular and structural elements of the human spleen. J Histochem Cytochem. 1972 Dec;20(12):1049–1058. doi: 10.1177/20.12.1049. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Hardy R., Pesando J. M., Schlossman S. F., Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981 Jan;67(1):134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTERSEN J. C. A COMPARISON OF THE METALOPHILIC RETICULOENDOTHELIAL CELLS TO CELLS CONTAINING ACID PHOSPHATASE AND NON-SPECIFIC ESTERASE IN THE LYMPHOID NODULES OF NORMAL AND STIMULATED RAT SPLEENS. Anat Rec. 1964 Jun;149:269–277. doi: 10.1002/ar.1091490209. [DOI] [PubMed] [Google Scholar]
- Ree H., Fanger H. Paracortical alteration in lymphadenopathic and tumor-draining lymph nodes: histologic study. Hum Pathol. 1975 May;6(3):363–372. doi: 10.1016/s0046-8177(75)80098-0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- SCOTHORNE R. J., MCGREGOR I. A. Cellular changes in lymph nodes and spleen following skin homografting in the rabbit. J Anat. 1955 Jul;89(3):283–292. [PMC free article] [PubMed] [Google Scholar]
- Sainte-Marie G., Peng F. S. Structural and cell population changes in the lymph nodes of the athymic nude mouse. Lab Invest. 1983 Oct;49(4):420–429. [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Takasaki S., Sakata A., Muroya T., Suzuki T., Ishikawa E. Lymphocyte subsets in the white pulp of human spleen in normal and diseased cases. Acta Pathol Jpn. 1984 Mar;34(2):251–270. doi: 10.1111/j.1440-1827.1984.tb07554.x. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- van Rooijen N., Kors N., van Nieuwmegen R. Double immunocytochemical evidence for a clonal development of specific antibody-containing cells in the rabbit spleen. Anat Rec. 1984 Jul;209(3):385–390. doi: 10.1002/ar.1092090318. [DOI] [PubMed] [Google Scholar]
- van Vliet E., Melis M., van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985 Jan;33(1):40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., De Wolf-Peeters C., Desmet V. J., Takahashi K., Ohtsuki Y., Akagi T. Nodular alteration of the paracortical area. An in situ immunohistochemical analysis of primary, secondary, and tertiary T-nodules. Am J Pathol. 1985 Jul;120(1):55–66. [PMC free article] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., De Vos R., Desmet V. J. Immature sinus histiocytosis. Light- and electron-microscopic features, immunologic phenotype, and relationship with marginal zone lymphocytes. Am J Pathol. 1985 Feb;118(2):266–277. [PMC free article] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., Vanstapel M. J., Desmet V. J. Improved immunohistochemical visualization of helper/inducer T-cells by the simultaneous application of two noncrossblocking monoclonal antibodies. Stain Technol. 1985 Jan;60(1):45–49. doi: 10.3109/10520298509113890. [DOI] [PubMed] [Google Scholar]
- van der Valk P., van der Loo E. M., Jansen J., Daha M. R., Meijer C. J. Analysis of lymphoid and dendritic cells in human lymph node, tonsil and spleen. A study using monoclonal and heterologous antibodies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):169–185. doi: 10.1007/BF02889863. [DOI] [PubMed] [Google Scholar]