Abstract
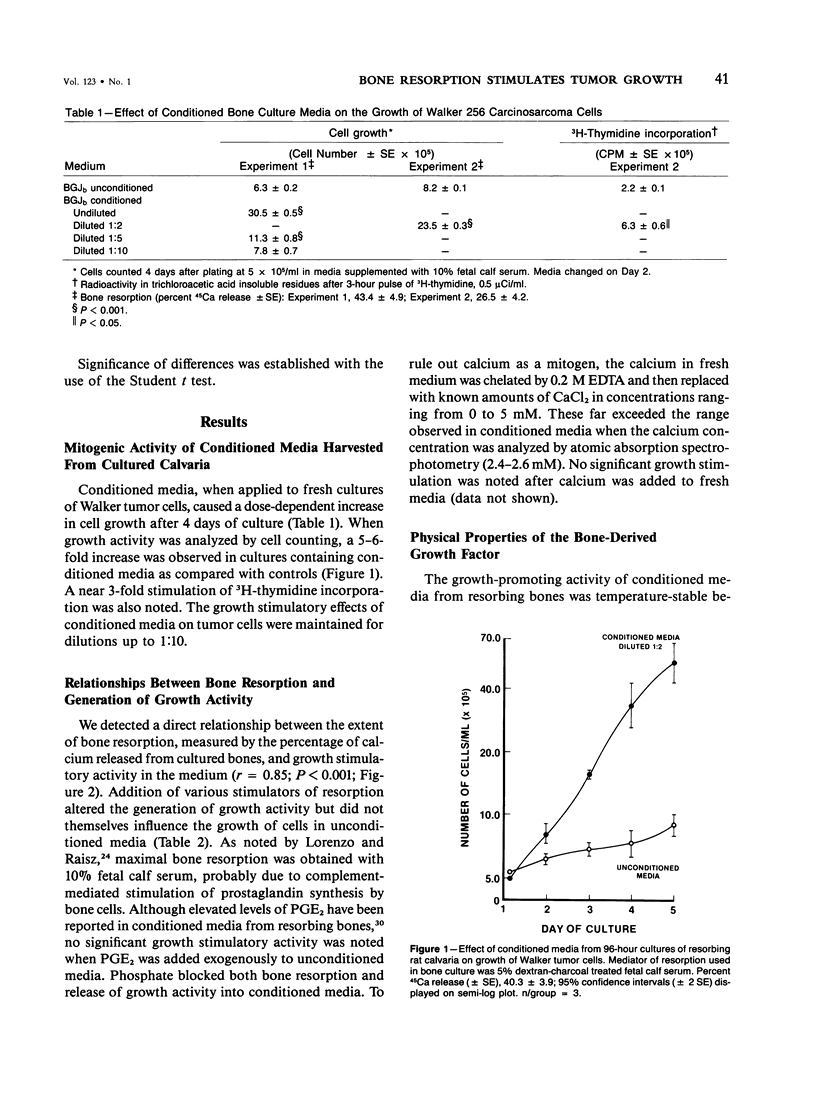
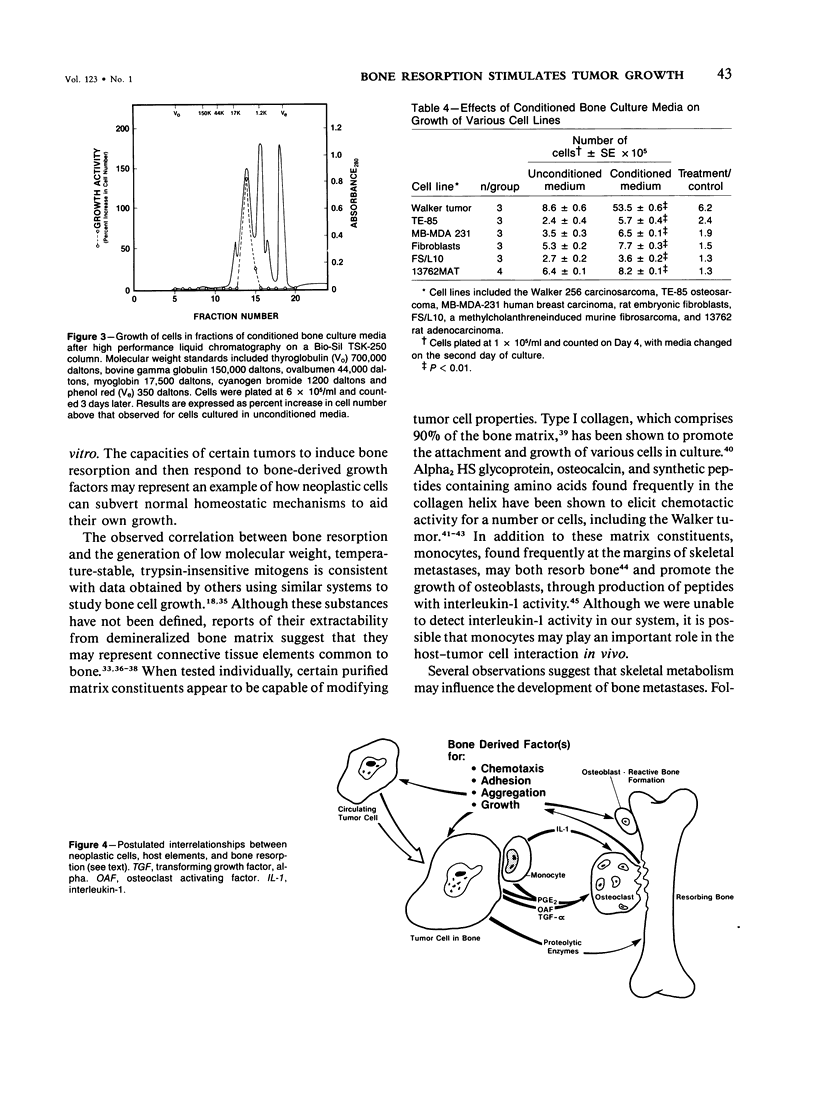
Demineralized extracts of bone matrix and conditioned media from cultured fetal rat calvaria have been reported to contain growth stimulatory activity for bone cells. To investigate the potential role of these local bone growth factors in the development of bone metastases, we chose the Walker 256 carcinosarcoma, a rat mammary tumor which causes osteolytic bone metastases and hypercalcemia. 45Ca-labeled, 19-day fetal Sprague-Dawley rat calvaria were cultured for 96 hours in BGJb medium. Walker cells from ascites tumors or cultures were grown in unconditioned media or in conditioned media harvested from the bone cultures, in the presence of 10% fetal calf serum. Media were changed every 2 days, cells were counted daily for 5 days, and 3H-thymidine uptake into acid insoluble residues was measured. The growth of tumor cells was 5-6-fold greater in conditioned media than in unconditioned media and the effect was dose dependent. Cells cultured in conditioned media demonstrated a approximately 3-fold enhancement of 3H-thymidine incorporation. Generation of growth stimulatory activity correlated with the extent of bone resorption, measured by release of 45Ca from the fetal parietal bones (r = 0.85; P less than 0.001). Conditioned media from bones cultured with 10(-7) M prostaglandin E2 (PGE2) contained greater amounts of growth stimulatory activity than untreated conditioned media, but PGE2 itself did not stimulate tumor cell growth. Addition of 3.5 mM PO4 to bone cultures blocked bone resorption and the generation of growth factors. Growth stimulatory activity was stable to heat (56 C for 30 minutes) and trypsin digestion, with an apparent molecular weight of less than 17,000 daltons by high-performance liquid chromatography. Conditioned medium also stimulated the growth of 13762 rat mammary adenocarcinoma cells, MB-MDA-231 human breast carcinoma cells, TE-85 osteosarcoma cells, a murine fibrosarcoma and rat embryonic fibroblasts, with the most potent effects noted for Walker tumor cells, the TE-85 osteosarcoma, and human breast carcinoma lines. These results suggest a mechanism by which bone resorption could promote the development of skeletal metastasis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canalis E. Effect of growth factors on bone cell replication and differentiation. Clin Orthop Relat Res. 1985 Mar;(193):246–263. [PubMed] [Google Scholar]
- Canalis E., Peck W. A., Raisz L. G. Stimulation of DNA and collagen synthesis by autologous growth factor in cultured fetal rat calvaria. Science. 1980 Nov 28;210(4473):1021–1023. doi: 10.1126/science.7434011. [DOI] [PubMed] [Google Scholar]
- Carter R. L. A role for local osteoclasts in determining the differential susceptibility of human cartilage and bone to invasion by carcinoma. Diagn Histopathol. 1982 Jul-Sep;5(3):213–217. [PubMed] [Google Scholar]
- Drivdahl R. H., Howard G. A., Baylink D. J. Extracts of bone contain a potent regulator of bone formation. Biochim Biophys Acta. 1982 Jan 12;714(1):26–33. doi: 10.1016/0304-4165(82)90123-4. [DOI] [PubMed] [Google Scholar]
- Drivdahl R. H., Puzas J. E., Howard G. A., Baylink D. J. Regulation of DNA synthesis in chick calvaria cells by factors from bone organ culture. Proc Soc Exp Biol Med. 1981 Nov;168(2):143–150. doi: 10.3181/00379727-168-41249. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Quantitative characteristics of the transformation of hamster cells by PARA (defective simian virus 40)-adenovirus 7. J Virol. 1970 May;5(5):568–577. doi: 10.1128/jvi.5.5.568-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E., Mundy G. R. Relation of osteoclast activating factor production to extent of bone disease in multiple myeloma. Br J Haematol. 1981 Jan;47(1):21–30. doi: 10.1111/j.1365-2141.1981.tb02758.x. [DOI] [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Association of increased cyclic adenosine 3':5'-monophosphate content in cultured human breast cancer cells and release of hydrolytic enzymes and bone-resorbing activity. Cancer Res. 1983 Dec;43(12 Pt 1):5792–5794. [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978 Dec 14;276(5689):726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- Farley J. R., Masuda T., Wergedal J. E., Baylink D. J. Human skeletal growth factor: characterization of the mitogenic effect on bone cells in vitro. Biochemistry. 1982 Jul 6;21(14):3508–3513. doi: 10.1021/bi00257a038. [DOI] [PubMed] [Google Scholar]
- Frisch B., Bartl R., Mahl G., Burkhardt R. Scope and value of bone marrow biopsies in metastatic cancer. Invasion Metastasis. 1984;4 (Suppl 1):12–30. [PubMed] [Google Scholar]
- Gabel D., von Hofsten B. Some properties of a bacterial proteinase chemically fixed to agarose. Eur J Biochem. 1970 Sep;15(3):410–414. doi: 10.1111/j.1432-1033.1970.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Galasko C. S. Mechanisms of lytic and blastic metastatic disease of bone. Clin Orthop Relat Res. 1982 Sep;(169):20–27. [PubMed] [Google Scholar]
- Galasko C. S. The pathological basis for skeletal scintigraphy. J Bone Joint Surg Br. 1975 Aug;57(3):353–359. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Guaitani A., Polentarutti N., Filippeschi S., Marmonti L., Corti F., Italia C., Coccioli G., Donelli M. G., Mantovani A., Garattini S. Effects of disodium etidronate in murine tumor models. Eur J Cancer Clin Oncol. 1984 May;20(5):685–693. doi: 10.1016/0277-5379(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Hart I. R. 'Seed and soil' revisited: mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982;1(1):5–16. doi: 10.1007/BF00049477. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Fidler I. J. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980 Jul;40(7):2281–2287. [PubMed] [Google Scholar]
- Hirshorn J. E., Vrhovsek E., Posen S. Carcinoma of the breast associated with hypercalcemia and the presence of parathyroid hormone-like substances in the tumor. J Clin Endocrinol Metab. 1979 Feb;48(2):217–221. doi: 10.1210/jcem-48-2-217. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Jung A., Bornand J., Mermillod B., Edouard C., Meunier P. J. Inhibition by diphosphonates of bone resorption induced by the Walker tumor of the rat. Cancer Res. 1984 Jul;44(7):3007–3011. [PubMed] [Google Scholar]
- Kalinowski D. T., Goodwin C. A. Case report 179: Metastatic disease developing in Paget disease of bone in the distal end of femur. Skeletal Radiol. 1981;7(3):229–231. doi: 10.1007/BF00361873. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. H., Lee M. Y., Linkhart T. A., Mohan S., Vermeiden J., Liu C. C., Baylink D. J. A mouse tumor-derived osteolytic factor stimulates bone resorption by a mechanism involving local prostaglandins production in bone. Biochim Biophys Acta. 1985 May 29;840(1):56–68. doi: 10.1016/0304-4165(85)90162-x. [DOI] [PubMed] [Google Scholar]
- Magro C., Orr F. W., Manishen W. J., Sivananthan K., Mokashi S. S. Adhesion, chemotaxis, and aggregation of Walker carcinosarcoma cells in response to products of resorbing bone. J Natl Cancer Inst. 1985 Apr;74(4):829–838. [PubMed] [Google Scholar]
- Malone J. D., Teitelbaum S. L., Griffin G. L., Senior R. M., Kahn A. J. Recruitment of osteoclast precursors by purified bone matrix constituents. J Cell Biol. 1982 Jan;92(1):227–230. doi: 10.1083/jcb.92.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P. J., Travers R. Continuous infusion of 1,25-dihydroxyvitamin D3 stimulates bone turnover in the normal young mouse. Calcif Tissue Int. 1983 Jul;35(4-5):418–425. doi: 10.1007/BF02405070. [DOI] [PubMed] [Google Scholar]
- McDonald D. F., Schofield B. H., Prezioso E. M., Adams V. L., Frondoza C. A., Trivedi S. M., Craig C., Humphrey R. L. Direct bone resorbing activity of murine myeloma cells. Cancer Lett. 1983 Jun;19(2):119–124. doi: 10.1016/0304-3835(83)90145-3. [DOI] [PubMed] [Google Scholar]
- Minne H., Raue F., Bellwinkel S., Ziegler R. The hypercalcaemic syndrome in rats bearing the Walker carcinosarcoma 256. Acta Endocrinol (Copenh) 1975 Mar;78(3):613–624. doi: 10.1530/acta.0.0780613. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., DeMartino S., Rowe D. W. Collagen and collagen-derived fragments are chemotactic for tumor cells. J Clin Invest. 1981 Oct;68(4):1102–1105. doi: 10.1172/JCI110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy G. R., Poser J. W. Chemotactic activity of the gamma-carboxyglutamic acid containing protein in bone. Calcif Tissue Int. 1983;35(2):164–168. doi: 10.1007/BF02405025. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Delikatny J., Jain N., Ward P. A. Comparison of the chemotactic responsiveness of two fibrosarcoma subpopulations of differing malignancy. Am J Pathol. 1981 Feb;102(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- Orr W., Varani J., Gondex M. K., Ward P. A., Mundy G. R. Chemotactic responses of tumor cells to products of resorbing bone. Science. 1979 Jan 12;203(4376):176–179. doi: 10.1126/science.569363. [DOI] [PubMed] [Google Scholar]
- Powell N. Metastatic carcinoma in association with Paget's disease of bone. Br J Radiol. 1983 Aug;56(668):582–585. doi: 10.1259/0007-1285-56-668-582. [DOI] [PubMed] [Google Scholar]
- Puzas J. E., Drivdahl R. H., Howard G. A., Baylink D. J. Endogenous inhibitor of bone cell proliferation. Proc Soc Exp Biol Med. 1981 Jan;166(1):113–122. doi: 10.3181/00379727-166-41032. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Sandberg A. L., Goodson J. M., Simmons H. A., Mergenhagen S. E. Complement-dependent stimulation of prostaglandin synthesis and bone resorption. Science. 1974 Aug 30;185(4153):789–791. doi: 10.1126/science.185.4153.789. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Simmons H. A., Gworek S. C., Eilon G. Studies on congenital osteopetrosis in microphthalmic mice using organ cultures: impairment of bone resorption in response to physiologic stimulators. J Exp Med. 1977 Apr 1;145(4):857–865. doi: 10.1084/jem.145.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath N. C., Reddi A. H. Collagenous bone matrix is a local mitogen. Nature. 1979 Apr 26;278(5707):855–857. doi: 10.1038/278855a0. [DOI] [PubMed] [Google Scholar]
- Rifas L., Shen V., Mitchell K., Peck W. A. Macrophage-derived growth factor for osteoblast-like cells and chondrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4558–4562. doi: 10.1073/pnas.81.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Rodan G. A., Simmons H. A., Walenga R. W., Feinstein M. B., Raisz L. G. Bone resorptive factor produced by osteosarcoma cells with osteoblastic features is PGE2. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1358–1365. doi: 10.1016/s0006-291x(81)80161-1. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., DeSimone D. P., Reddi A. H. Extracellular bone matrix-derived growth factor. Exp Cell Res. 1982 Dec;142(2):460–464. doi: 10.1016/0014-4827(82)90389-5. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Carter C. S., Katz S. I., Oppenheim J. J. Epidermal cell production of thymocyte activating factor (ETAF). J Invest Dermatol. 1982 Jul;79(1):34–39. doi: 10.1111/1523-1747.ep12510569. [DOI] [PubMed] [Google Scholar]
- Stern P. H., Miller J. C., Chen S. F., Kahn D. J. A bone resorbing substance from bovine serum albumin (brA). Calcif Tissue Res. 1978 Aug 18;25(3):233–240. doi: 10.1007/BF02010775. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Role of prostaglandins in the production of hypercalcemia by tumors. Cancer Res. 1978 Nov;38(11 Pt 2):4138–4141. [PubMed] [Google Scholar]
- Tsao S. W., Burman J. F., Pittam M. R., Carter R. L. Further observations on mechanisms of bone destruction by squamous carcinomas of the head and neck: the role of host stroma. Br J Cancer. 1983 Nov;48(5):697–704. doi: 10.1038/bjc.1983.252. [DOI] [PMC free article] [PubMed] [Google Scholar]


