Abstract
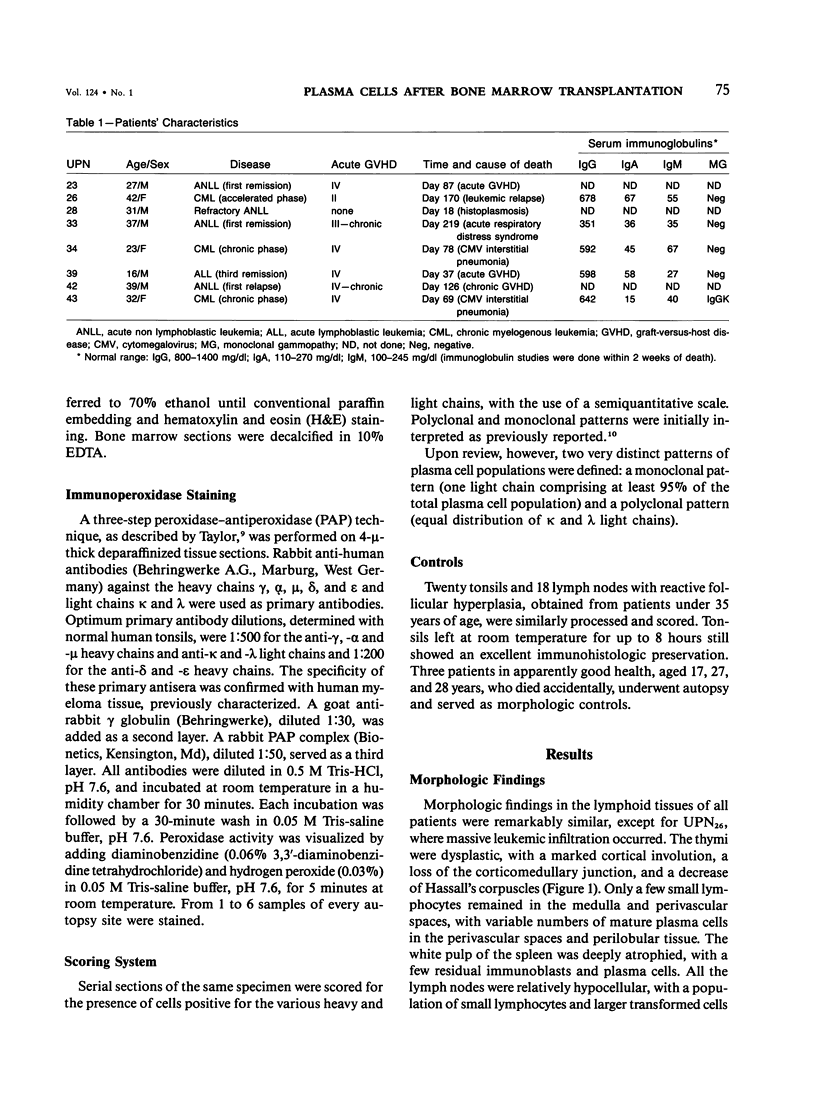
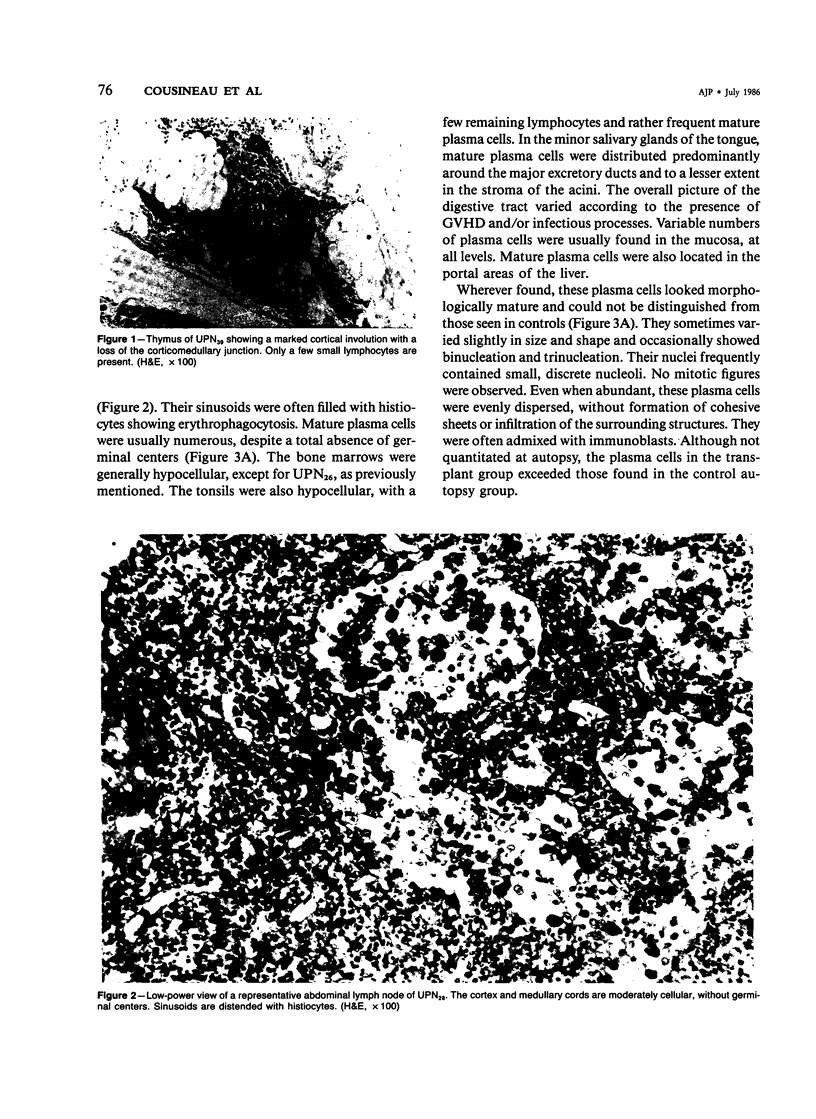
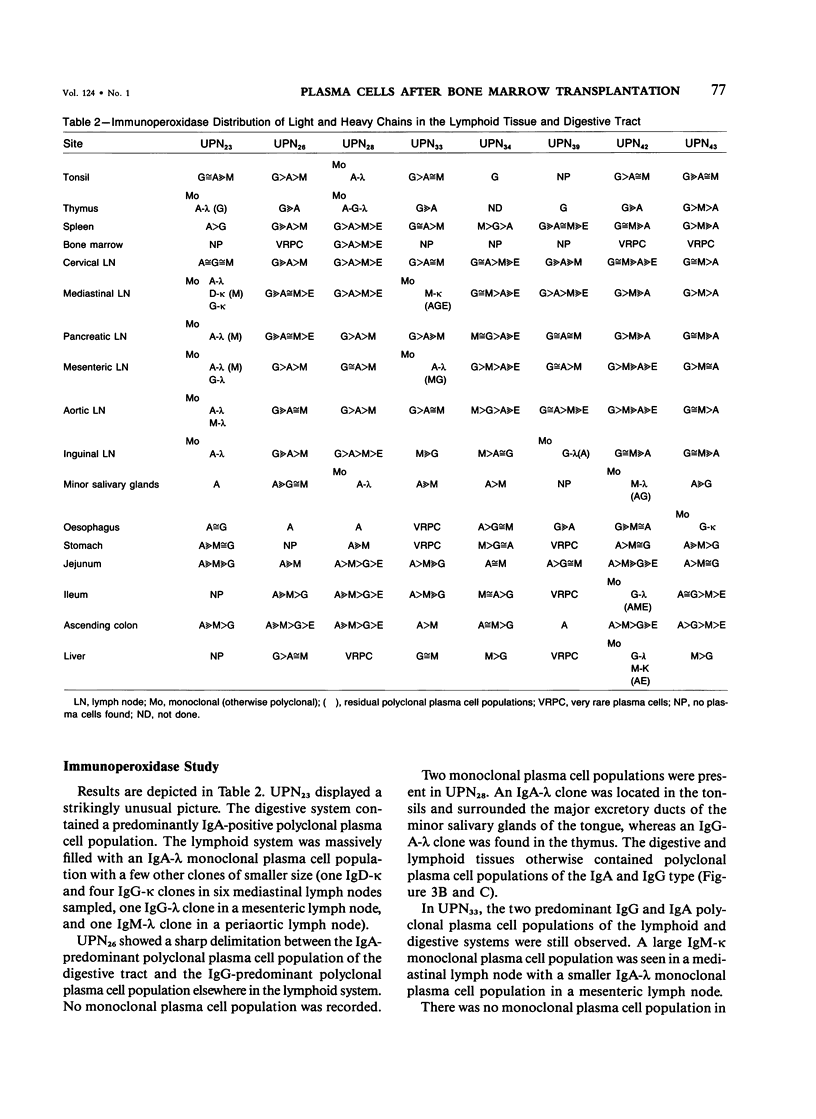
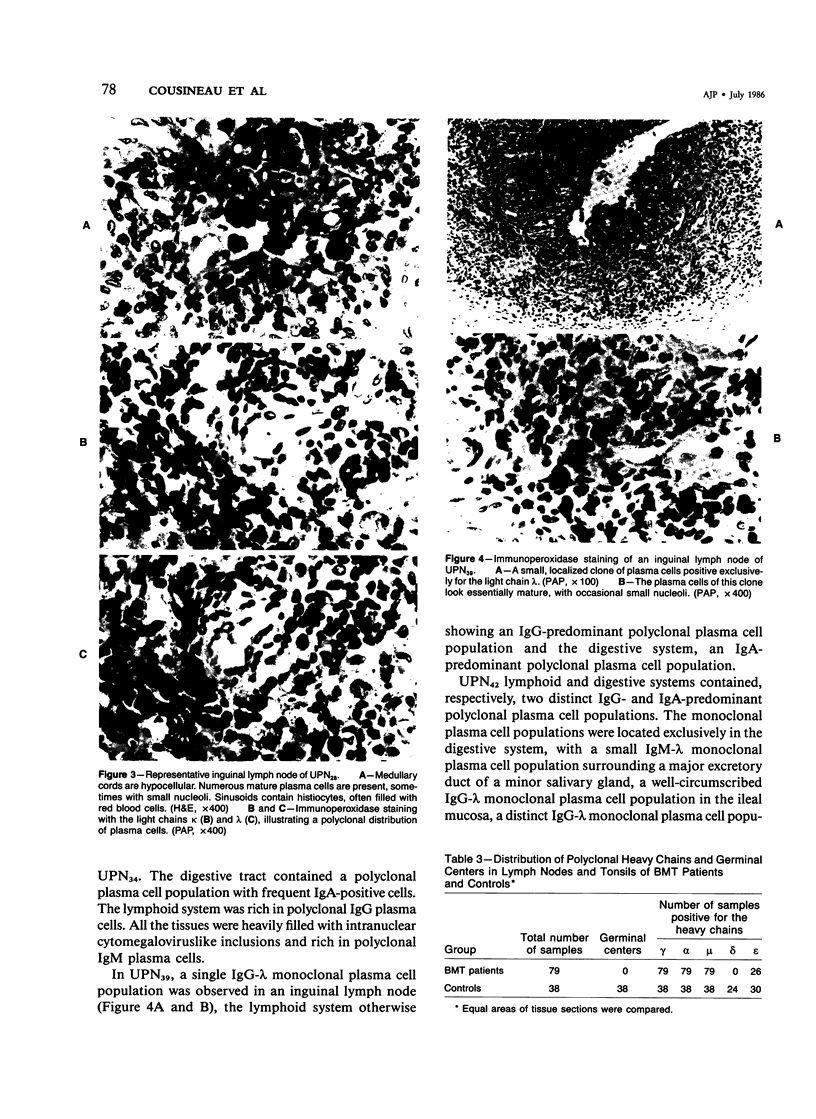
Postmortem fixed tissue sections of the lymphoid and digestive systems of eight consecutive leukemic patients dying of various diseases after bone marrow transplantation (BMT) were analyzed for the presence of the heavy chains gamma, alpha, mu, delta, and epsilon and light chains kappa and lambda, with the use of a standard immunoperoxidase method. Two distinct types of plasma cell populations were found. The first type was a widely distributed polyclonal plasma cell population, lacking IgD-positive plasma cells and germinal centers. The second type of plasma cell population, found in 6 of 8 patients, was a group of monoclonal plasma cell populations positive for the heavy chains gamma, alpha, mu, or delta. Recent immunohistologic observations of the human lymph node suggest that the first type of polyclonal plasma cell population could arise from a nonspecific expansion of sIgM+, sIgD- B lymphocytes. The lack of germinal centers, a structure closely involved in specific-antibody production, may correlate with the poor specific-antibody response documented in patients after BMT. The monoclonal plasma cell populations, found with an unexpectedly high frequency, are probably related to a functional T-cell defect.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beschorner W. E., Hutchins G. M., Elfenbein G. J., Santos G. W. The thymus in patients with allogeneic bone marrow transplants. Am J Pathol. 1978 Jul;92(1):173–186. [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Nadler L. M., Stashenko P., McCluskey R. T., Schlossman S. F. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981 Sep 1;154(3):737–749. doi: 10.1084/jem.154.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg H. J., Storb R., Thomas E. D. Bone marrow transplantation: a review of delayed complications. Br J Haematol. 1984 Jun;57(2):185–208. [PubMed] [Google Scholar]
- Fireman P., Johnson H. A., Gitlin D. Presence of plasma cells and gamma-1-M-globulin synthesis in a patient with thymic alymphoplasia. Pediatrics. 1966 Mar;37(3):485–492. [PubMed] [Google Scholar]
- Graze P. R., Gale R. P. Chronic graft versus host disease: a syndrome of disordered immunity. Am J Med. 1979 Apr;66(4):611–620. doi: 10.1016/0002-9343(79)91171-9. [DOI] [PubMed] [Google Scholar]
- Greipp P. R., Kyle R. A. Clinical, morphological, and cell kinetic differences among multiple myeloma, monoclonal gammopathy of undetermined significance, and smoldering multiple myeloma. Blood. 1983 Jul;62(1):166–171. [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 1. Identification with monoclonal antibodies in normal lymphoid tissues. Am J Pathol. 1984 Mar;114(3):387–395. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 2. Immunoglobulin expression of germinal center cells. Am J Pathol. 1984 Mar;114(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. B., Caporale L. H., Thorbecke G. J. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol. 1974 Sep;13(3):416–430. doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Kunkl A. The role of germinal centres in the generation of immunological memory. Ciba Found Symp. 1981;84:265–280. doi: 10.1002/9780470720660.ch14. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Elfenbein G. J., Goldman C. K., Marshall S. L., Santos G. W., Waldmann T. A. B cell, helper T cell, and suppressor T cell abnormalities contribute to disordered immunoglobulin synthesis in patients following bone marrow transplantation. Transplantation. 1982 Feb;33(2):184–190. doi: 10.1097/00007890-198202000-00015. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Gagnon R. F., Smart Y. Selective loss of large lymphocytes from the marginal zone of the white pulp in rat spleens following a single dose of cyclophosphamide. A study using quantitative histological methods. Immunology. 1980 May;40(1):123–131. [PMC free article] [PubMed] [Google Scholar]
- Kumararatne D. S., MacLennan I. C. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol. 1981 Nov;11(11):865–869. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- Layton J. E., Baker J., Bartlett P. F., Shortman K. Antigen-initiated B lymphocyte differentiation. XVIII. Pre-progenitor B cells that give primary adoptive responses are s-IgM+IgD-Ia+. J Immunol. 1981 Apr;126(4):1227–1233. [PubMed] [Google Scholar]
- Levy N., Nelson J., Meyer P., Lukes R. J., Parker J. W. Reactive lymphoid hyperplasia with single class (monoclonal) surface immunoglobulin. Am J Clin Pathol. 1983 Sep;80(3):300–308. doi: 10.1093/ajcp/80.3.300. [DOI] [PubMed] [Google Scholar]
- Noel D. R., Witherspoon R. P., Storb R., Atkinson K., Doney K., Mickelson E. M., Ochs H. D., Warren R. P., Weiden P. L., Thomas E. D. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978 Jun;51(6):1087–1105. [PubMed] [Google Scholar]
- Perreault C., Gyger M., Boileau J., Bonny Y., Cousineau S., Lacombe M., Lavallee R., Tawil E., D'Angelo G. Acute graft-versus-host disease after allogeneic bone marrow transplantation. Can Med Assoc J. 1983 Nov 1;129(9):969–974. [PMC free article] [PubMed] [Google Scholar]
- Perreault C., Pelletier M., Landry D., Gyger M. Study of Langerhans cells after allogeneic bone marrow transplantation. Blood. 1984 Apr;63(4):807–811. [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radl J., Mink J. G., van den Berg P., van Zwieten M. J., Benner R. Increased frequency of homogeneous immunoglobulins in the sera of nude athymic mice with age. Clin Immunol Immunopathol. 1980 Nov;17(3):469–476. doi: 10.1016/0090-1229(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Radl J., de Glopper E., van den Berg P., van Zwieten M. J. Idiopathic paraproteinemia. III. Increased frequency of paraproteinemia in thymectomized aging C57BL/KaLwRij and CBA/BrARij mice. J Immunol. 1980 Jul;125(1):31–35. [PubMed] [Google Scholar]
- Ringden O., Witherspoon R. P., Storb R., Ekelund E., Thomas E. D. Increased in vitro B-cell IgG secretion during acute graft-versus-host disease and infection. Observations in 50 human marrow transplant recipients. Blood. 1980 Feb;55(2):179–186. [PubMed] [Google Scholar]
- Rádl J., Dooren L. J., Eijsvoogel V. P., van Went J. J., Hijmans W. An immunological study during post-transplantation follow-up of a case of severe combined immunodeficiency. Clin Exp Immunol. 1972 Feb;10(2):367–382. [PMC free article] [PubMed] [Google Scholar]
- Rádl J., van den Berg P., Voormolen M., Hendriks W. D., Schaefer U. W. Homogeneous immunoglobulins in sera of rhesus monkeys after lethal irradiation and bone marrow transplantation. Clin Exp Immunol. 1974 Feb;16(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Shortman K., Howard M., Teale J., Baker J. Antigen-initiated B lymphocyte differentiation. XVI. Primary and secondary adoptive responses involve two sequential stages, antigen nonspecific then antigen specific, in the generation of specific AFC. J Immunol. 1979 Jun;122(6):2465–2472. [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Thomas E., Storb R., Clift R. A., Fefer A., Johnson F. L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975 Apr 17;292(16):832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- White R. G., French V. I., Stark J. M. A study of the localisation of a protein antigen in the chicken spleen and its relation to the formation of germinal centres. J Med Microbiol. 1970 Feb;3(1):65–83. doi: 10.1099/00222615-3-1-65. [DOI] [PubMed] [Google Scholar]
- Witherspoon R. P., Lum L. G., Storb R. Immunologic reconstitution after human marrow grafting. Semin Hematol. 1984 Jan;21(1):2–10. [PubMed] [Google Scholar]
- Witherspoon R. P., Lum L. G., Storb R., Thomas E. D. In vitro regulation of immunoglobulin synthesis after human marrow transplantation. II. Deficient T and non-T lymphocyte function within 3-4 months of allogeneic, syngeneic, or autologous marrow grafting for hematologic malignancy. Blood. 1982 Apr;59(4):844–850. [PubMed] [Google Scholar]
- Witherspoon R. P., Storb R., Ochs H. D., Fluornoy N., Kopecky K. J., Sullivan K. M., Deeg J. H., Sosa R., Noel D. R., Atkinson K. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981 Aug;58(2):360–368. [PubMed] [Google Scholar]
- van Rooijen N., Kors N., van Nieuwmegen R. Double immunocytochemical evidence for a clonal development of specific antibody-containing cells in the rabbit spleen. Anat Rec. 1984 Jul;209(3):385–390. doi: 10.1002/ar.1092090318. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., De Vos R., Desmet V. J. Immature sinus histiocytosis. Light- and electron-microscopic features, immunologic phenotype, and relationship with marginal zone lymphocytes. Am J Pathol. 1985 Feb;118(2):266–277. [PMC free article] [PubMed] [Google Scholar]