Abstract
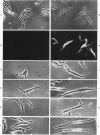
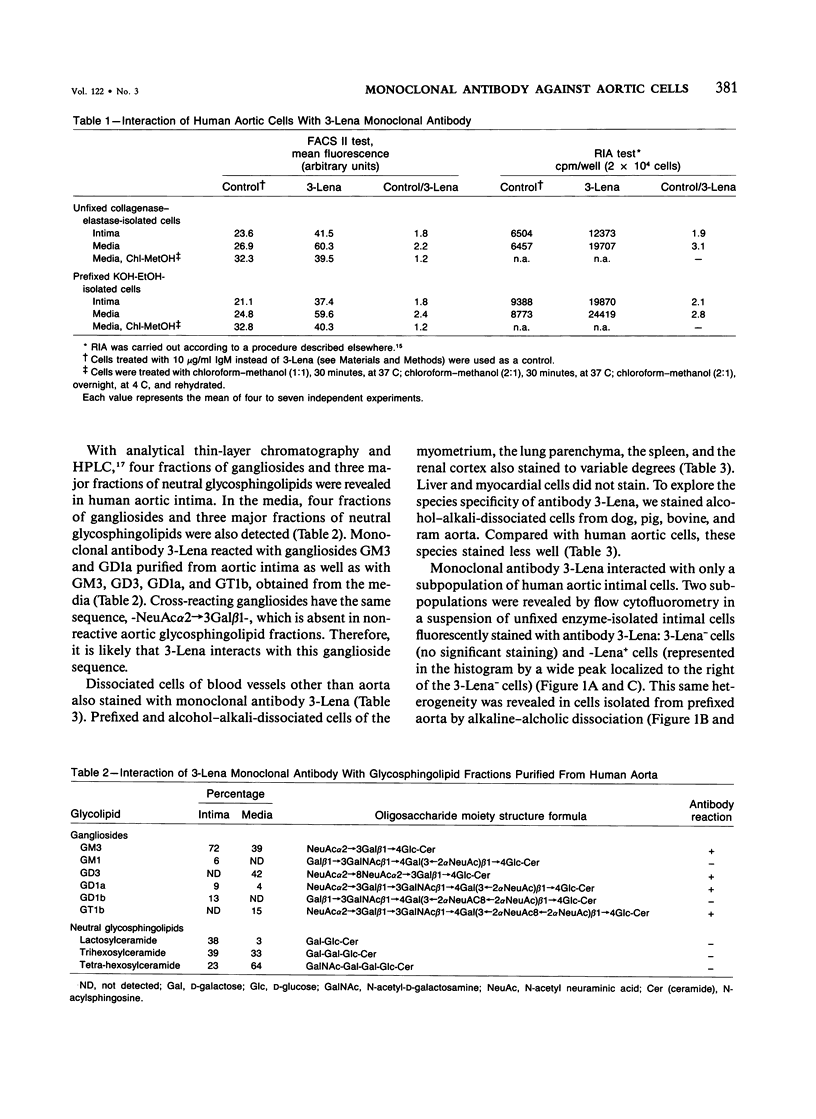
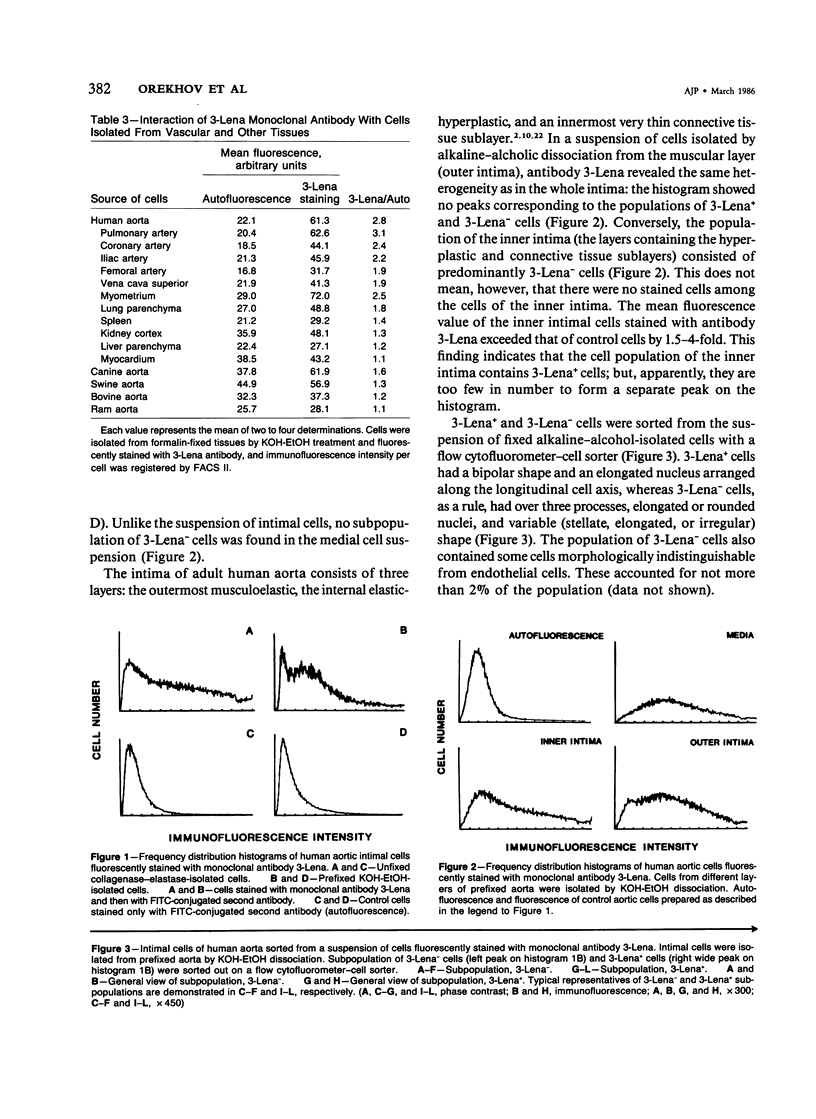
The cells of the arterial wall are heterogeneous. To study the functions and peculiarities of various subpopulations of arterial cells the authors have generated an IgM mouse monoclonal antibody, designated 3-Lena, which interacts with human aortic cells (demonstrated by RIA and flow cytofluorometry). The cellular antigens interacting with monoclonal antibody 3-Lena are gangliosides GM3, GD3, GD1a, and GT1b. Presumably, the epitope is represented by the structure -NeuAc alpha 2----3Gal beta 1, a component of the oligosaccharide moiety of the ganglioside molecule. In addition to aortic cells, those dissociated from other large human vessels as well as cells of the myometrium and lung parenchyma interact with 3-Lena. Cells dissociated from the spleen and renal cortex exhibit a substantially weaker interaction, while liver and myocardial cells do not react. Compared with human aortic cells, aortic cells of other mammalian species stain less effectively (dog, swine) or do not stain at all (bovine, ram). Flow cytofluorometric analysis demonstrates that practically all human aorta medial cells interact with antibody 3-Lena, whereas a certain portion of cells in the intimal population do not. In the outer intimal sublayer adjoining the media, the cells reacting with monoclonal antibody 3-Lena make up the bulk of the cell population, and the inner sublayers are characterized by a prevalence of nonreactive cells. After separation of reactive and nonreactive human aortic intimal cells by a cytofluorometer-cell sorter, 3-Lena+ cells were found to have an elongated bipolar shape, whereas most 3-Lena- cells have multiple processes and variable elongated, stellate, or irregular shapes.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF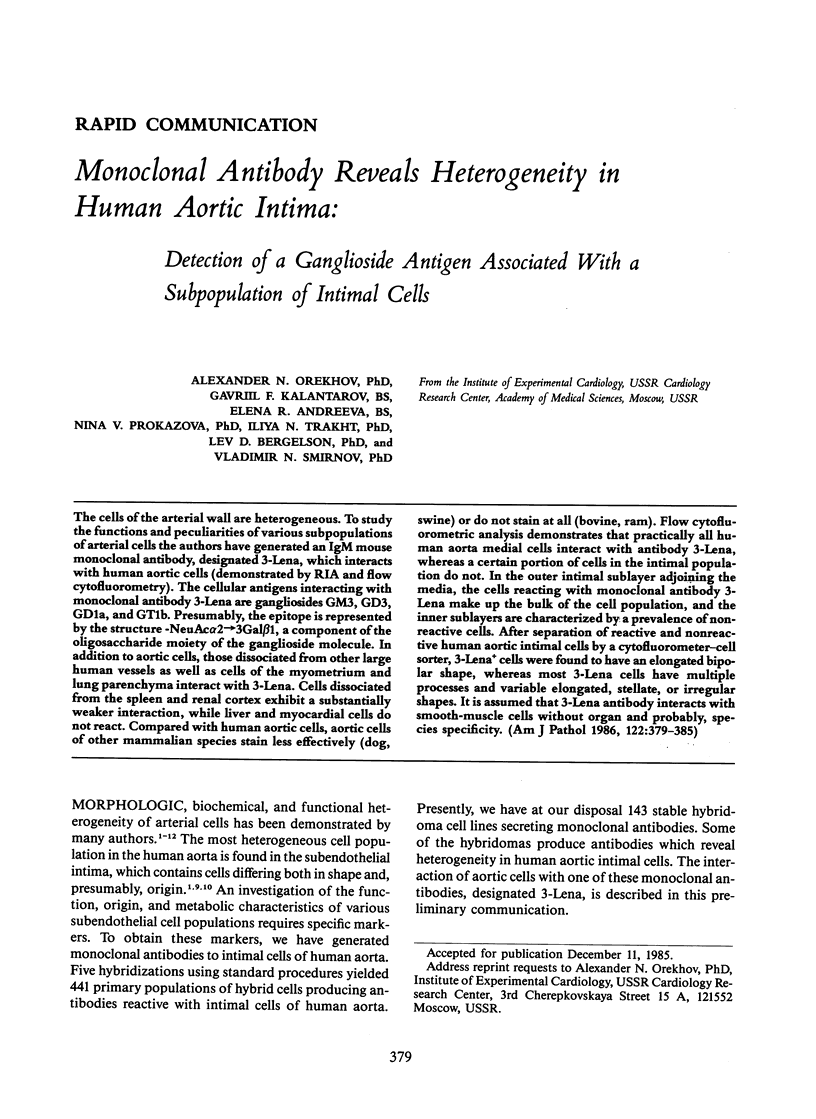

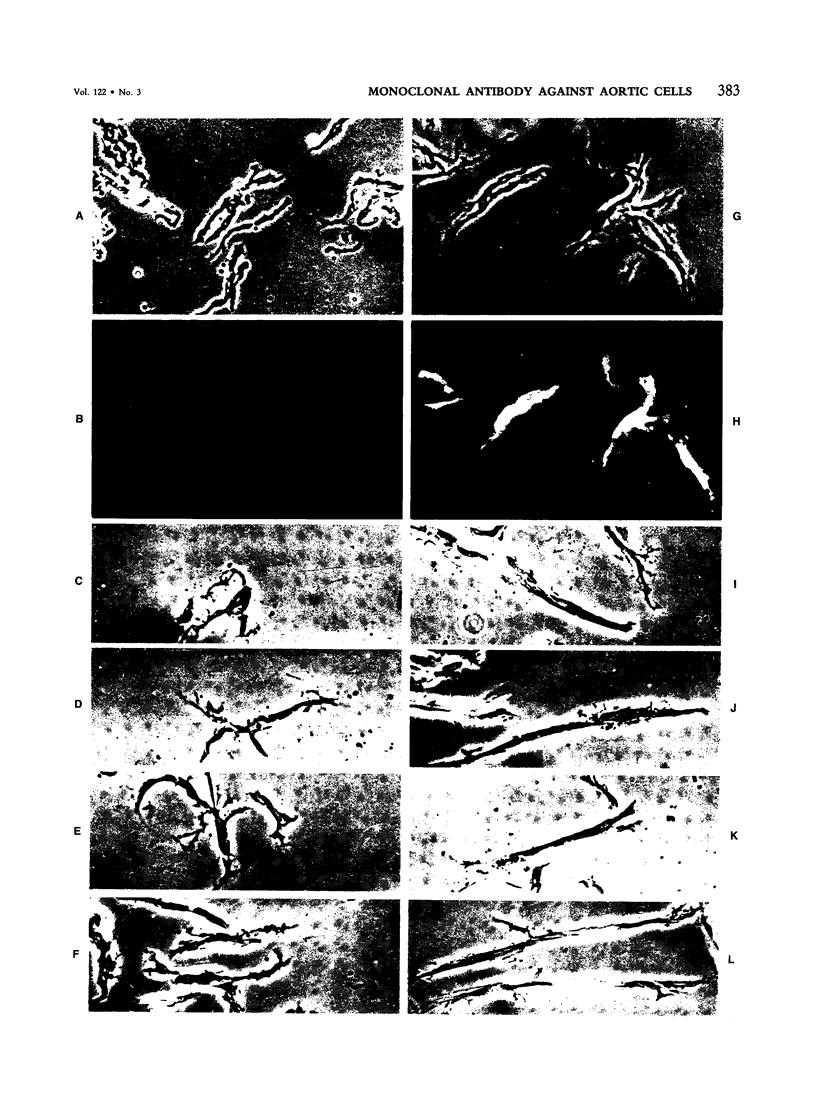


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feigl W., Sinzinger H., Howanietz L., Leithner C. A morphologically different type of smooth muscle cell in the inner media of the splenic artery. Acta Anat (Basel) 1976;94(4):617–625. doi: 10.1159/000144593. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Rungger-Brändle E., de Chastonay C., Franke W. W. Vimentin-containing smooth muscle cells in aortic intimal thickening after endothelial injury. Lab Invest. 1982 Sep;47(3):265–269. [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Grünwald J., Schäper W., Mey J., Hauss W. H. Special characteristics of cultured smooth muscle cell subtypes of hypertensive and diabetic rats. Artery. 1982;11(1):1–14. [PubMed] [Google Scholar]
- Imai H., Connell C. E., Lee K. T., Kim D. N., Thomas W. A. Differential counts by electron microscopy of cell types in normal intimal cell masses in swine abdominal aortas. Exp Mol Pathol. 1985 Jun;42(3):377–388. doi: 10.1016/0014-4800(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Mammary carcinoma: enzymatic block in disialoganglioside biosynthesis. Science. 1973 Nov 20;182(4115):935–937. doi: 10.1126/science.182.4115.935. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Fujiwara K., Alexander R. W., Gimbrone M. A., Jr Heterogeneity of myosin antigenic expression in vascular smooth muscle in vivo. Lab Invest. 1984 Apr;50(4):401–407. [PubMed] [Google Scholar]
- Lee K. T., Lee K. J., Lee S. K., Imai H., O'Neal R. M. Poorly differentiated subendothelial cells in swine aortas. Exp Mol Pathol. 1970 Aug;13(1):118–129. doi: 10.1016/0014-4800(70)90089-4. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., MORE R. H., HAUST M. D. The diffuse intimal thickening of the human aorta with aging. Am J Pathol. 1958 Nov-Dec;34(6):1023–1031. [PMC free article] [PubMed] [Google Scholar]
- O'Neal R. M. Derivation of intimal smooth muscle cells in normal arteries and atherosclerotic plaques. An overview. Prog Biochem Pharmacol. 1977;13:69–72. [PubMed] [Google Scholar]
- Orekhov A. N., Andreeva E. R., Tertov V. V., Krushinsky A. V. Dissociated cells from different layers of adult human aortic wall. Acta Anat (Basel) 1984;119(2):99–105. doi: 10.1159/000145868. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Karpova I. I., Tertov V. V., Rudchenko S. A., Andreeva E. R., Krushinsky A. V., Smirnov V. N. Cellular composition of atherosclerotic and uninvolved human aortic subendothelial intima. Light-microscopic study of dissociated aortic cells. Am J Pathol. 1984 Apr;115(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Orekhov A. N., Kosykh V. A., Novikov I. D., Rudchenko S. A., Krushinsky A. V., Smirnov V. N. Heterogeneity of human aortic cells in regard to RNA content. Blood Vessels. 1984;21(6):290–297. doi: 10.1159/000158531. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Kosykh V. A., Repin V. S., Smirnov V. N. Cell proliferation in normal and atherosclerotic human aorta. I. Flow cytofluorometric determination of cellular deoxyribonucleic acid content. Lab Invest. 1983 Apr;48(4):395–398. [PubMed] [Google Scholar]
- Orekhov A. N., Novikov I. D., Kalantarov G. F. Primary RIA screening of hybridoma supernatants without use of a negative control. J Immunol Methods. 1985 Mar 18;77(2):343–345. doi: 10.1016/0022-1759(85)90047-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Prokazova N. V., Kocharov S. L., Shaposhnikova G. I., Zvezdina N. D., Bergelson L. D. Changes of glycolipids dependent on cell density of Ehrlich ascites carcinoma cells. Eur J Biochem. 1984 Jun 15;141(3):527–529. doi: 10.1111/j.1432-1033.1984.tb08224.x. [DOI] [PubMed] [Google Scholar]
- Rokhlin O. V., Petrosyan M. N., Ibraghimov A. R., Vengerova T. I., Bogachova G. T. Antigenic markers of V domain of mouse MOPC 21 IgG1 L chain. The conformational basis of VL domain markers and content of MOPC 21 VL-like immunoglobulins in sera of inbred mouse strains. Eur J Immunol. 1983 May;13(5):397–403. doi: 10.1002/eji.1830130509. [DOI] [PubMed] [Google Scholar]
- Saito M., Yu R. K., Cheung N. K. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem Biophys Res Commun. 1985 Feb 28;127(1):1–7. doi: 10.1016/s0006-291x(85)80117-0. [DOI] [PubMed] [Google Scholar]
- Schönfelder M. Orthologie und Pathologie der Langhans-Zellen der Aortenintima des Menschen. Pathol Microbiol (Basel) 1969;33(3):129–145. [PubMed] [Google Scholar]
- Sinzinger H., Silberbauer K., Auerswald W. Does prostacyclin (PGI2) regulate human arterial intima smooth muscle cell proliferation in early atherogenesis? Blood Vessels. 1980;17(1):58–60. doi: 10.1159/000158235. [DOI] [PubMed] [Google Scholar]
- Sternberg J., Jeppesen P. Dot-blotting--a novel screening assay for antibodies in hybridoma cultures. J Immunol Methods. 1983 Nov 11;64(1-2):39–43. doi: 10.1016/0022-1759(83)90382-4. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Arao Y. A new solvent system for the separation of neutral glycosphingolipids. J Lipid Res. 1981 Aug;22(6):1020–1024. [PubMed] [Google Scholar]
- Yates A. J., Thompson D. K., Boesel C. P., Albrightson C., Hart R. W. Lipid composition of human neural tumors. J Lipid Res. 1979 May;20(4):428–436. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]