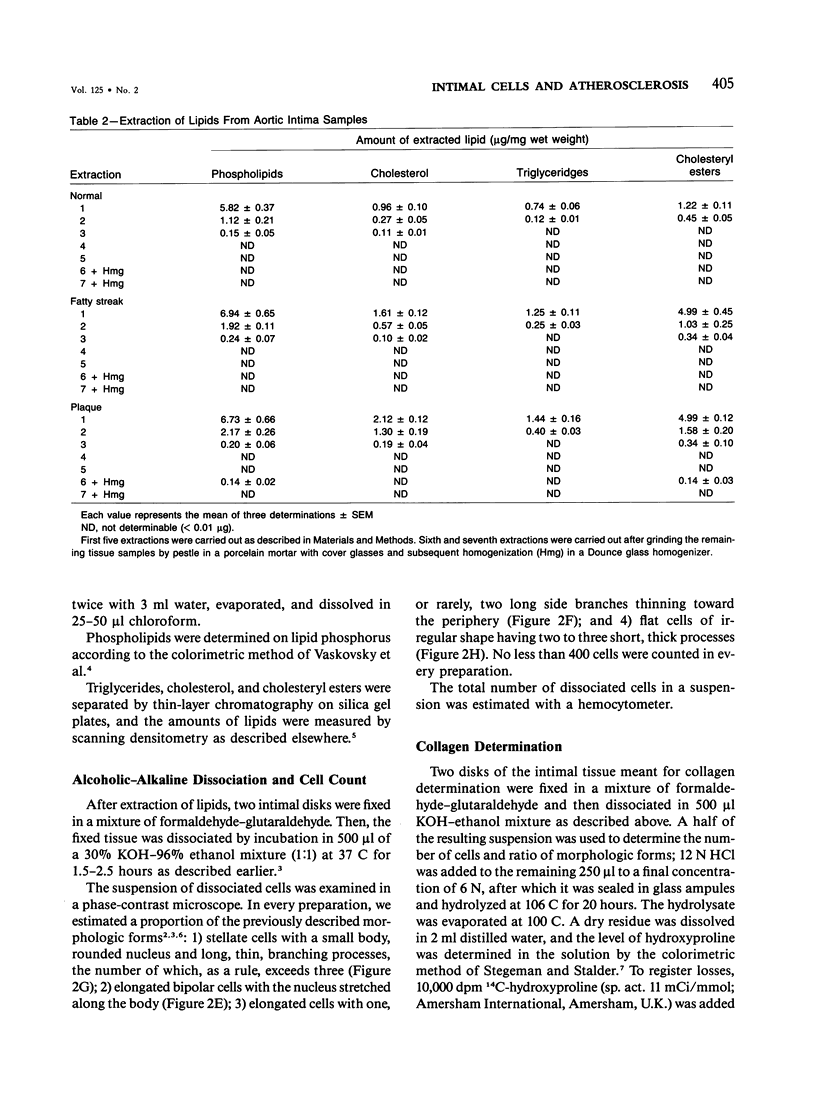
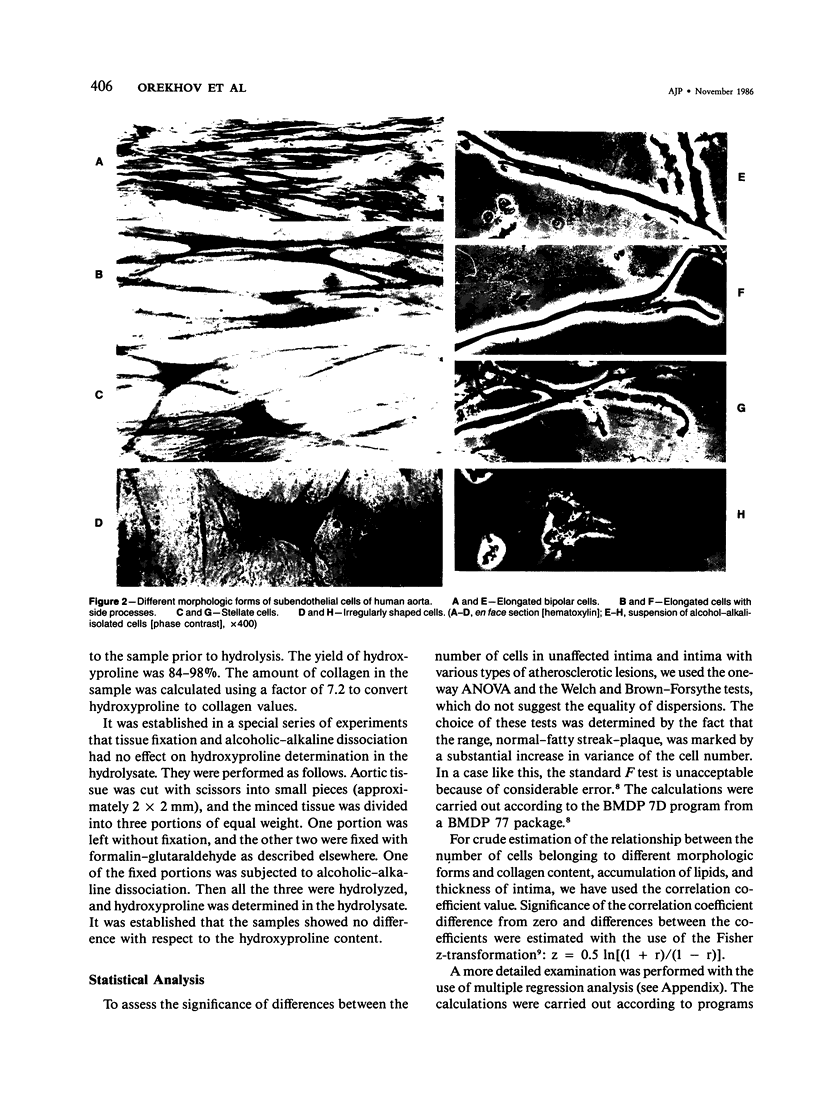
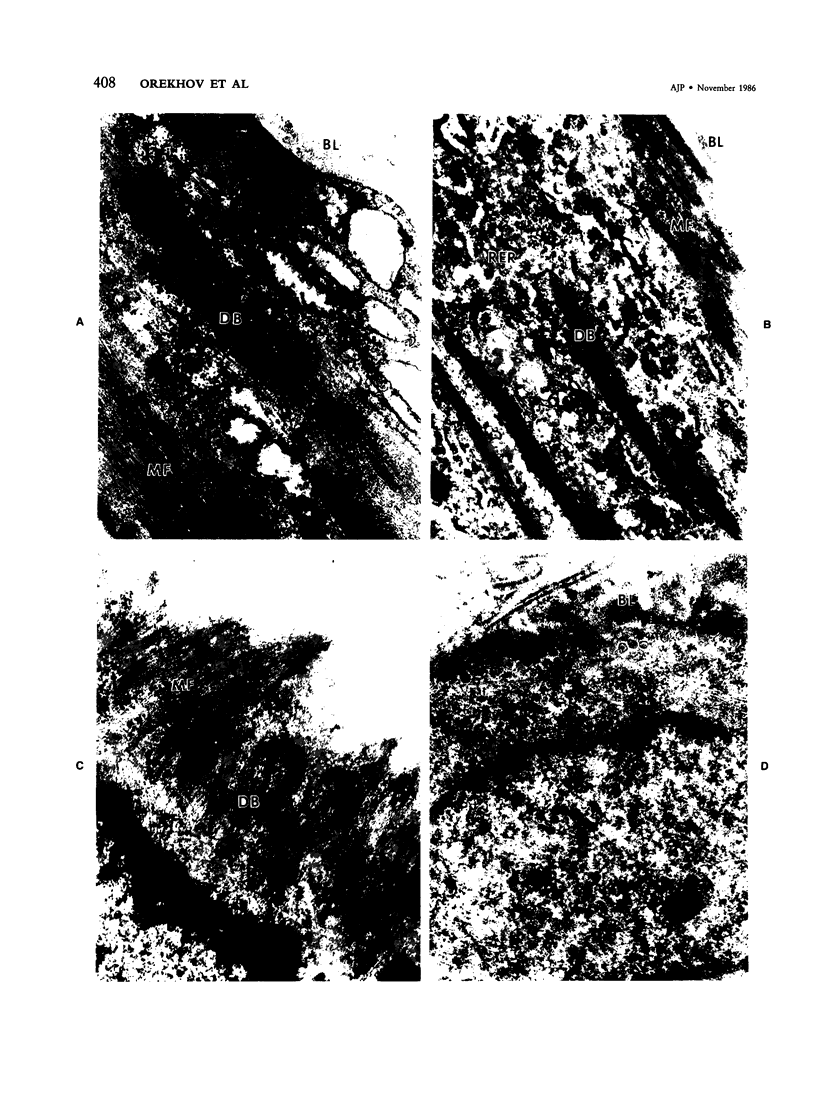
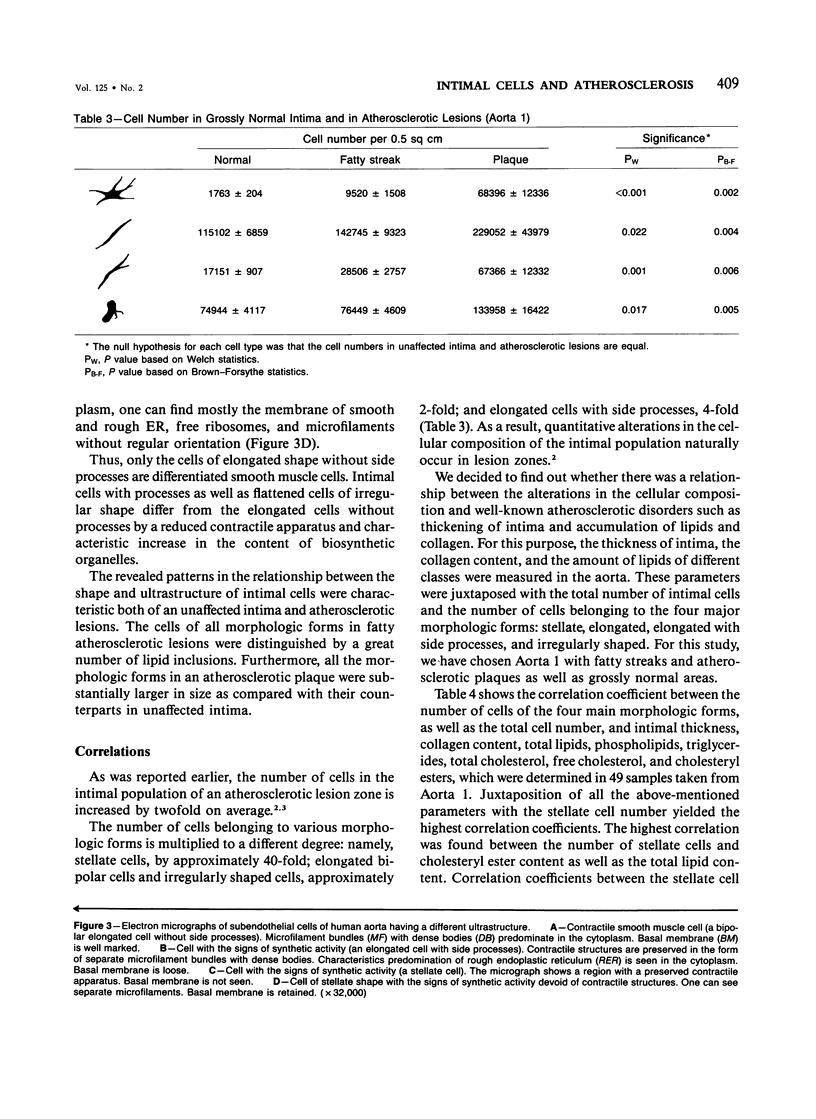
Abstract
The subendothelial intima of human aorta is populated by cells of various shapes. Round and ovoid cells which are lymphocyte- and monocyte-like hematogenous cells account for less than 5% of the cell population. The bulk of the intimal population (over 95%) is made up of cells that can be described as elongated, stellate, elongated with side processes, and irregularly shaped. To identify these morphologic forms, the authors have used target electron microscopy. It has been established that elongated cells devoid of side processes possess all the ultrastructural features of differentiated smooth muscle cells: a developed contractile apparatus in the form of microfilament bundles with dense bodies occupying most of the cytoplasm, basal membrane surrounding the whole of the cell, and micropinocytotic vesicles along the plasma membrane. The other morphologic forms have an ultra-structure that allows us to identify them as so-called modified smooth muscle cells. They differ from the typical smooth muscle cells in that they have fewer contractile structures and a more developed biosynthetic apparatus. Some of stellate and irregular shaped cells are utterly devoid of contractile structures. To quantitate the number of cells of different morphologic forms, the authors used alcoholic-alkaline dissociation of prefixed intima. It was established that the intimal population is multiplied at the site of an atherosclerotic lesion, the number of stellate cells being increased much more substantially, compared with other morphologic cell forms. It was found that an increase in the number of stellate cells is related to such sequelae of atherosclerosis in aorta as intimal thickening, deposition of lipids, and an increased amount of collagen. There was a high positive correlation between the alteration in the stellate cell number occurring in the intima and the above-mentioned parameters (correlation coefficients were 0.732, 0.800 and 0.953, respectively). The correlations between these indexes and the total number of intimal cells or the number of cells belonging to each of the other morphologic forms were not so high. A multivariate analysis gave similar results. Thus, it may be suggested that stellate cells are the principal cell type involved in the disease. This report discusses the origin of stellate and other intimal cells and their role in atherogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS J. U., HAUST M. D., MORE R. H. ELECTRON-MICROSCOPIC STUDIES IN HUMAN ATHEROSCLEROSIS; CELLULAR ELEMENTS IN AORTIC FATTY STREAKS. Exp Mol Pathol. 1964 Oct;90:511–525. doi: 10.1016/0014-4800(64)90031-0. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., RITCHIE A. C. The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol; together with a description of the anatomy of the intima of the rabbit's aorta and the spontaneous lesions which occur in it. Am J Pathol. 1957 Sep-Oct;33(5):845–873. [PMC free article] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960 Oct;37:377–389. [PMC free article] [PubMed] [Google Scholar]
- Issler R. W. The arterial medial cell, smooth muscle or multifunctional mesenchyme? J Atheroscler Res. 1968 Mar-Apr;8(2):201–213. doi: 10.1016/s0368-1319(68)80056-0. [DOI] [PubMed] [Google Scholar]
- KHAVKIN T. N. O razviti ateroskleroticheskikh izmenenii aorty. Arkh Patol. 1950 Sep-Oct;12(5):23–33. [PubMed] [Google Scholar]
- Katz S. S. The lipids or grossly normal human aortic intima from birth to old age. J Biol Chem. 1981 Dec 10;256(23):12275–12280. [PubMed] [Google Scholar]
- Kojimahara M., Yamazaki K., Ooneda G. Ultrastructural study of hemangiomas. 1. Capillary hemangioma of the skin. Acta Pathol Jpn. 1981 Jan;31(1):105–115. [PubMed] [Google Scholar]
- Krushinsky A. V., Orekhov A. N., Smirnov V. N. Stellate cells in the intima of human aorta. Application of alkaline dissociation method in the analysis of the vessel wall cellular content. Acta Anat (Basel) 1983;117(3):266–269. [PubMed] [Google Scholar]
- Lee K. T., Lee K. J., Lee S. K., Imai H., O'Neal R. M. Poorly differentiated subendothelial cells in swine aortas. Exp Mol Pathol. 1970 Aug;13(1):118–129. doi: 10.1016/0014-4800(70)90089-4. [DOI] [PubMed] [Google Scholar]
- O'Neal R. M. Derivation of intimal smooth muscle cells in normal arteries and atherosclerotic plaques. An overview. Prog Biochem Pharmacol. 1977;13:69–72. [PubMed] [Google Scholar]
- Orekhov A. N., Andreeva E. R., Tertov V. V., Krushinsky A. V. Dissociated cells from different layers of adult human aortic wall. Acta Anat (Basel) 1984;119(2):99–105. doi: 10.1159/000145868. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Karpova I. I., Tertov V. V., Rudchenko S. A., Andreeva E. R., Krushinsky A. V., Smirnov V. N. Cellular composition of atherosclerotic and uninvolved human aortic subendothelial intima. Light-microscopic study of dissociated aortic cells. Am J Pathol. 1984 Apr;115(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Orekhov A. N., Tertov V. V., Novikov I. D., Krushinsky A. V., Andreeva E. R., Lankin V. Z., Smirnov V. N. Lipids in cells of atherosclerotic and uninvolved human aorta. I. Lipid composition of aortic tissue and enzyme-isolated and cultured cells. Exp Mol Pathol. 1985 Feb;42(1):117–137. doi: 10.1016/0014-4800(85)90022-x. [DOI] [PubMed] [Google Scholar]
- PRIOR J. T., JONES D. B. Structural alterations within the aortic intima in infancy and childhood. Am J Pathol. 1952 Sep-Oct;28(5):937–951. [PMC free article] [PubMed] [Google Scholar]
- Parker F., Odland G. F. A correlative histochemical, biochemical and electron microscopic study of experimental atherosclerosis in the rabbit aorta with special reference to the myo-intimal cell. Am J Pathol. 1966 Feb;48(2):197–239. [PMC free article] [PubMed] [Google Scholar]
- Pietilä K., Nikkari T. Role of the arterial smooth muscle cell in the pathogenesis of atherosclerosis. Med Biol. 1983 Feb;61(1):31–44. [PubMed] [Google Scholar]
- Puchtler H., Sweat F., Terry M. S., Conner H. M. Investigation of staining, polarization and fluorescence-microscopic properties of myoendothelial cells. J Microsc. 1969;89(1):95–104. doi: 10.1111/j.1365-2818.1969.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- STILL W. J., O'NEAL R. M. Electron microscopic study of experimental atherosclerosis in the rat. Am J Pathol. 1962 Jan;40:21–35. [PMC free article] [PubMed] [Google Scholar]
- Sachs E. S. Effects of autolysis in vitro on the fine structure of human aortic intimal cells. J Atheroscler Res. 1967 Sep-Oct;7(5):549–565. doi: 10.1016/s0368-1319(67)80033-4. [DOI] [PubMed] [Google Scholar]
- Schönfelder M. Orthologie und Pathologie der Langhans-Zellen der Aortenintima des Menschen. Pathol Microbiol (Basel) 1969;33(3):129–145. [PubMed] [Google Scholar]
- Scott R. F., Jones R., Daoud A. S., Zumbo O., Coulston F., Thomas W. A. Experimental atherosclerosis in rhesus monkeys. II. Cellular elements of proliferative lesions and possible role of cytoplasmic degeneration in pathogenesis as studied by electron microscopy. Exp Mol Pathol. 1967 Aug;7(1):34–57. doi: 10.1016/0014-4800(67)90037-8. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Kubota I., Kamio A., Takagi T. Ultrastructural aspects of human atherosclerosis; role of the foam cells and modified smooth muscle cells. J Electron Microsc (Tokyo) 1972;21(4):301–313. [PubMed] [Google Scholar]
- Thomas W. A., Florentin R. A., Nam S. C., Kim D. N., Jones R. M., Lee K. T. Preproliferative phase of atherosclerosis in swine fed cholesterol. Arch Pathol. 1968 Dec;86(6):621–643. [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y., Vasendin I. M. A universal reagent for phospholipid analysis. J Chromatogr. 1975 Nov 12;114(1):129–141. doi: 10.1016/s0021-9673(00)85249-8. [DOI] [PubMed] [Google Scholar]




