Abstract
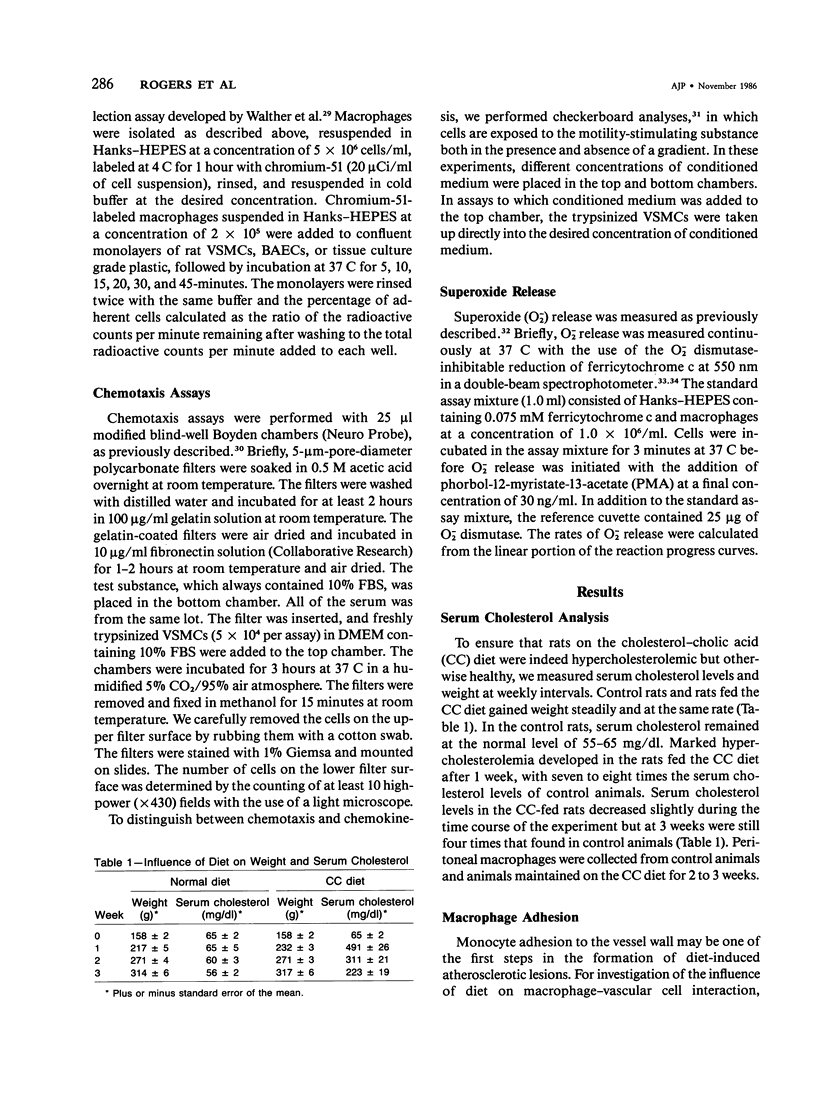
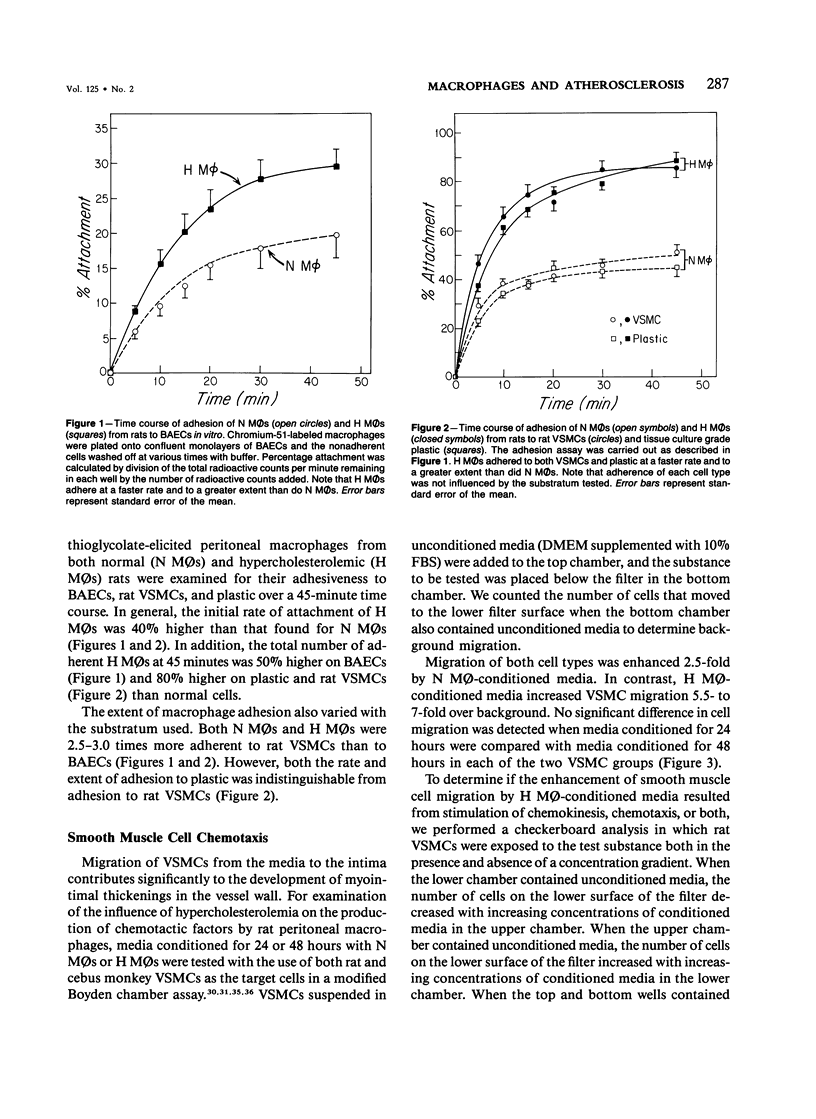
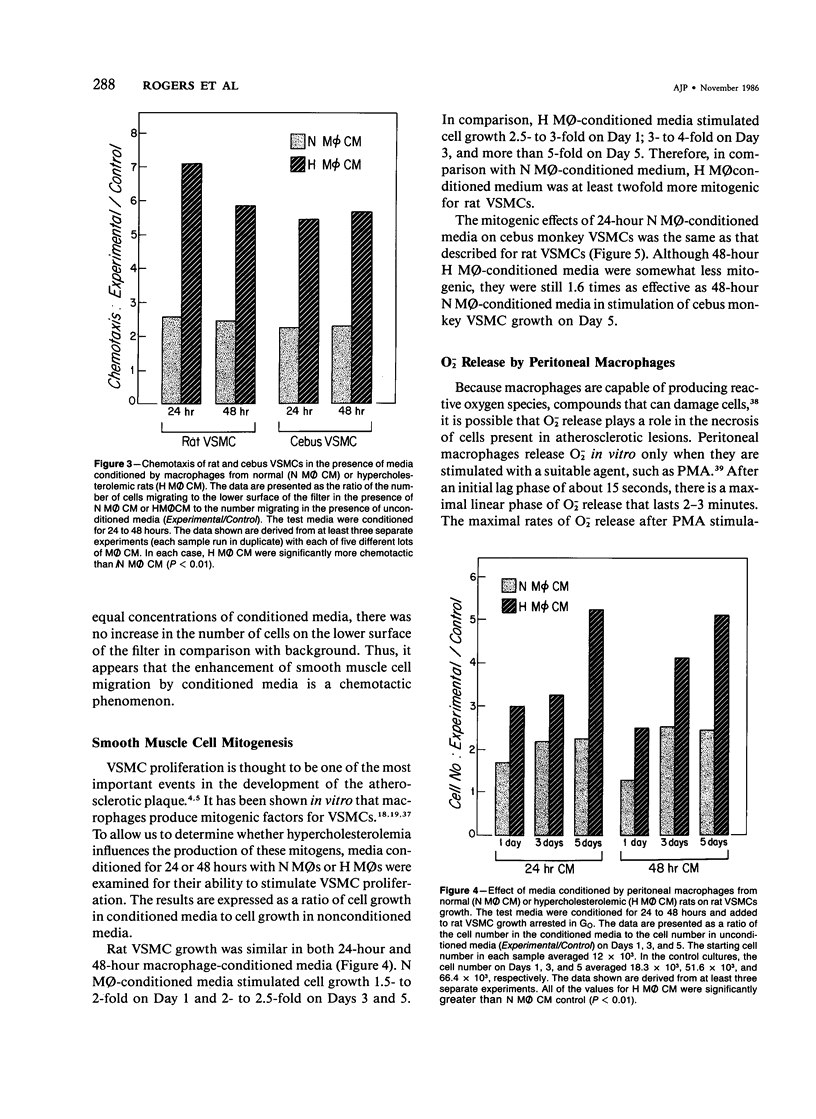
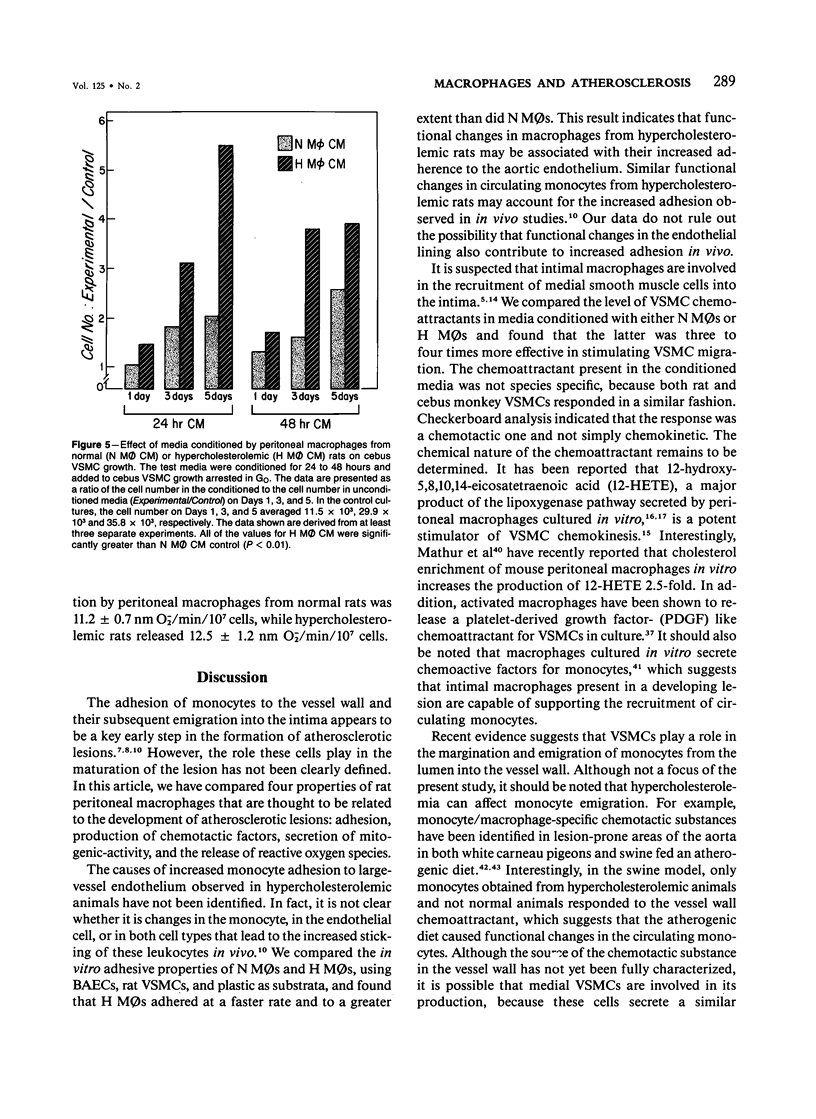
In diet-induced hypercholesterolemia, circulating monocytes adhere to the endothelium of the vessel wall and emigrate into the intima. Atherosclerotic lesions may develop, characterized by the presence of lipid-laden macrophages and proliferating smooth muscle cells recruited from the media. Using rat peritoneal macrophages, the authors examined the influence of diet-induced hypercholesterolemia on several variables of macrophage function that may contribute to lesion formation, including adhesion to bovine aortic endothelial cells (BAECs) and vascular smooth muscle cells (VSMCs), the production of chemoattractants and mitogens for VSMCs, and the release of the reactive oxygen species, superoxide. In general, a hypercholesterolemia-induced augmentation of macrophage function was observed. In comparison with macrophages from normal animals (N M phi s), macrophages from hypercholesterolemic animals (H M phi s) were 50-80% more adhesive to BAECs and VSMCs. H M phi-secreted products increased VSMC migration 6 to 7-fold, whereas N M0s only stimulated motility 2.5-fold. In addition, H M phi-conditioned media produced increased VSMC growth 5-fold, compared with a 2.5-fold increase produced by N M phi-conditioned media. Although the production of superoxide was found to be the same for both N M phi s and H M phi s, the release of superoxide by macrophages found in the intima of hypercholesterolemic animals may contribute to the necrosis of cells in the developing lesion. These results suggest that dietary cholesterol may accelerate atherosclerotic lesion formation by inducing specific changes in the properties of circulating monocytes and intimal macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Robinson J. M., Lazdins J. K., Briggs R. T., Karnovsky M. J., Karnovsky M. L. Comparative biochemical and cytochemical studies on superoxide and peroxide in mouse macrophages. J Cell Physiol. 1983 May;115(2):208–216. doi: 10.1002/jcp.1041150216. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Favreau L. V., Karnovsky M. J., Rosenberg R. D. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982 Oct 10;257(19):11256–11260. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Karnovsky M. J., Spiegelman B. M. Differentiation-dependent stimulation of neovascularization and endothelial cell chemotaxis by 3T3 adipocytes. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5597–5601. doi: 10.1073/pnas.79.18.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Karnovsky M. J., Spiegelman B. M. Potent stimulation of vascular endothelial cell growth by differentiated 3T3 adipocytes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6007–6011. doi: 10.1073/pnas.77.10.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Breslow J. L., Karnovsky M. J. Regression of myointimal thickening following carotid endothelial injury and development of aortic foam cell lesions in long term hypercholesterolemic rats. Lab Invest. 1977 Jan;36(1):73–81. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Ingerman-Wojenski C. M., Sedar A. W., Nissenbaum M., Silver M. J., Klurfeld D. M., Kritchevsky D. Early morphological changes in the endothelium of a peripheral artery of rabbits fed an atherogenic diet. Exp Mol Pathol. 1983 Feb;38(1):48–60. doi: 10.1016/0014-4800(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill H. C., Jr George Lyman Duff memorial lecture. Persistent problems in the pathogenesis of atherosclerosis. Arteriosclerosis. 1984 Sep-Oct;4(5):443–451. doi: 10.1161/01.atv.4.5.443. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch H., Durand J., Rigaud M., Mendy F., Breton J. C. Transformation of arachidonic acid into monohydroxy-eicosatetraenoic acids by mouse peritoneal macrophages. Lipids. 1981 Jul;16(7):518–524. doi: 10.1007/BF02535050. [DOI] [PubMed] [Google Scholar]
- Rigaud M., Durand J., Breton J. C. Transfomration of arachidonic acid into 12-hydroxy-5,8,10,14-eicosatetraenoic acid by mouse peritoneal macrophages. Biochim Biophys Acta. 1979 May 25;573(2):408–412. doi: 10.1016/0005-2760(79)90074-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Superoxide release by neutrophils: synergistic effects of a phorbol ester and a calcium ionophore. Biochem Biophys Res Commun. 1984 Jul 31;122(2):734–739. doi: 10.1016/s0006-291x(84)80095-9. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Sprague E. A., Kelley J. L., Valente A. J., Suenram C. A. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):175–191. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- Tvedten H. W., Till G. O., Ward P. A. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol. 1985 Apr;119(1):92–100. [PMC free article] [PubMed] [Google Scholar]
- Valente A. J., Fowler S. R., Sprague E. A., Kelley J. L., Suenram C. A., Schwartz C. J. Initial characterization of a peripheral blood mononuclear cell chemoattractant derived from cultured arterial smooth muscle cells. Am J Pathol. 1984 Dec;117(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Ziats N. P., Robertson A. L., Jr Effects of peripheral blood monocytes on human vascular cell proliferation. Atherosclerosis. 1981 Feb-Mar;38(3-4):401–410. doi: 10.1016/0021-9150(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


