Abstract
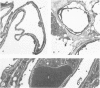
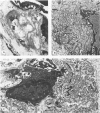
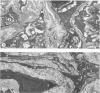
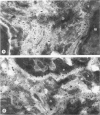
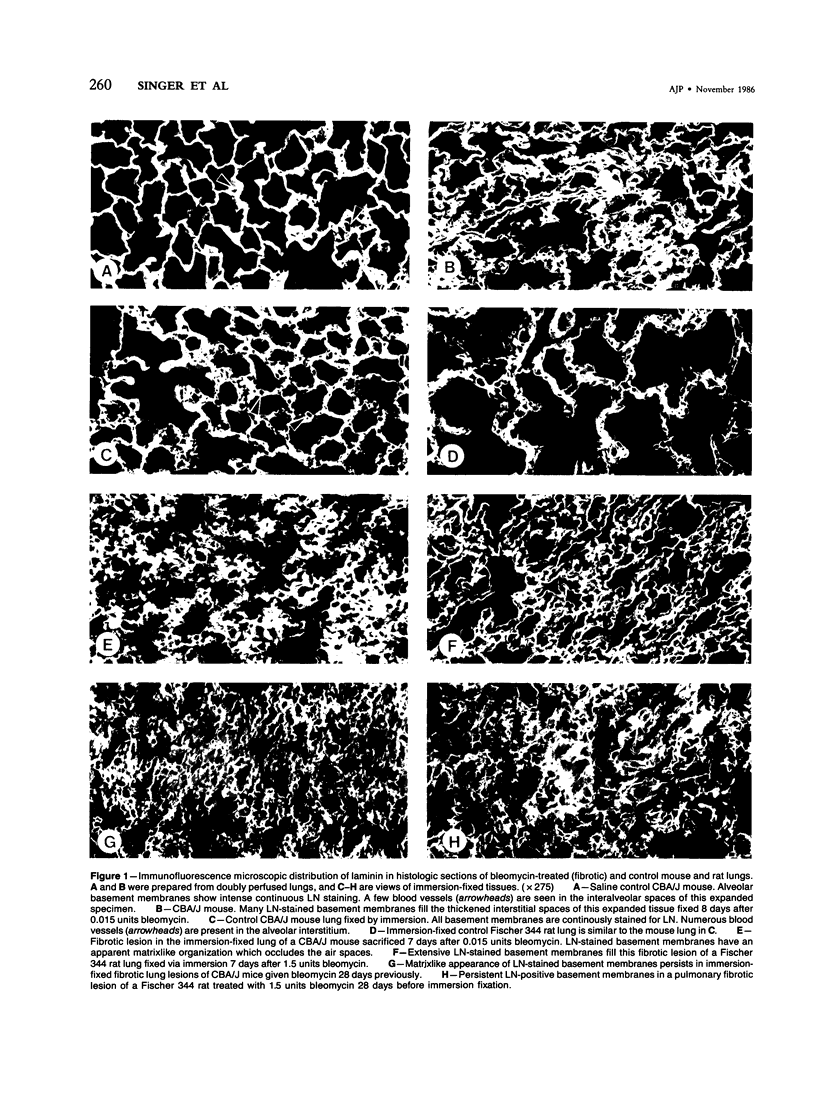
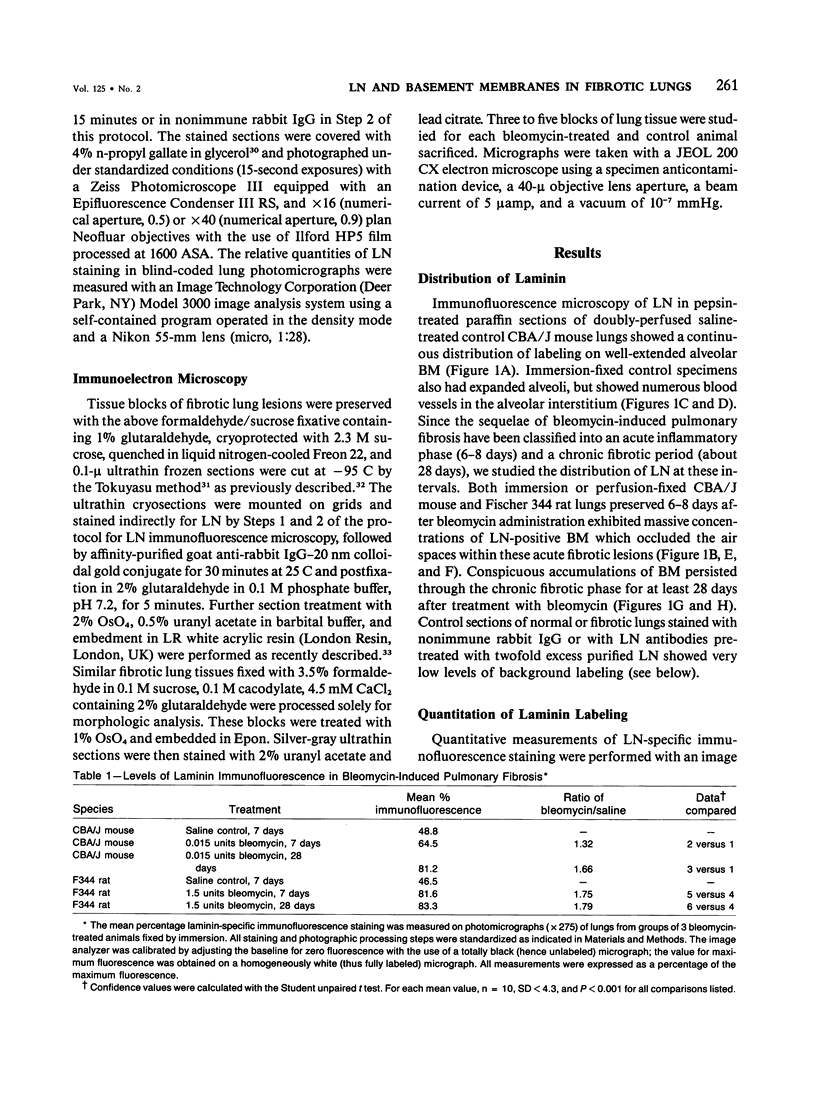
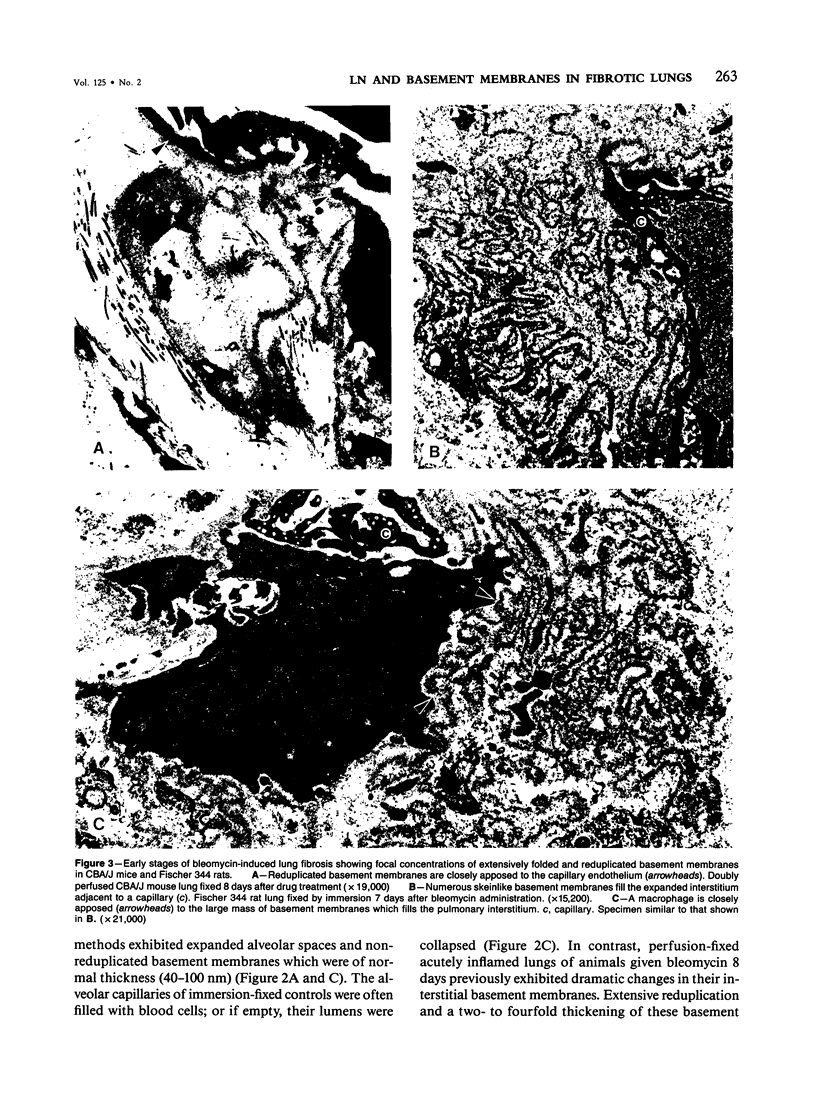
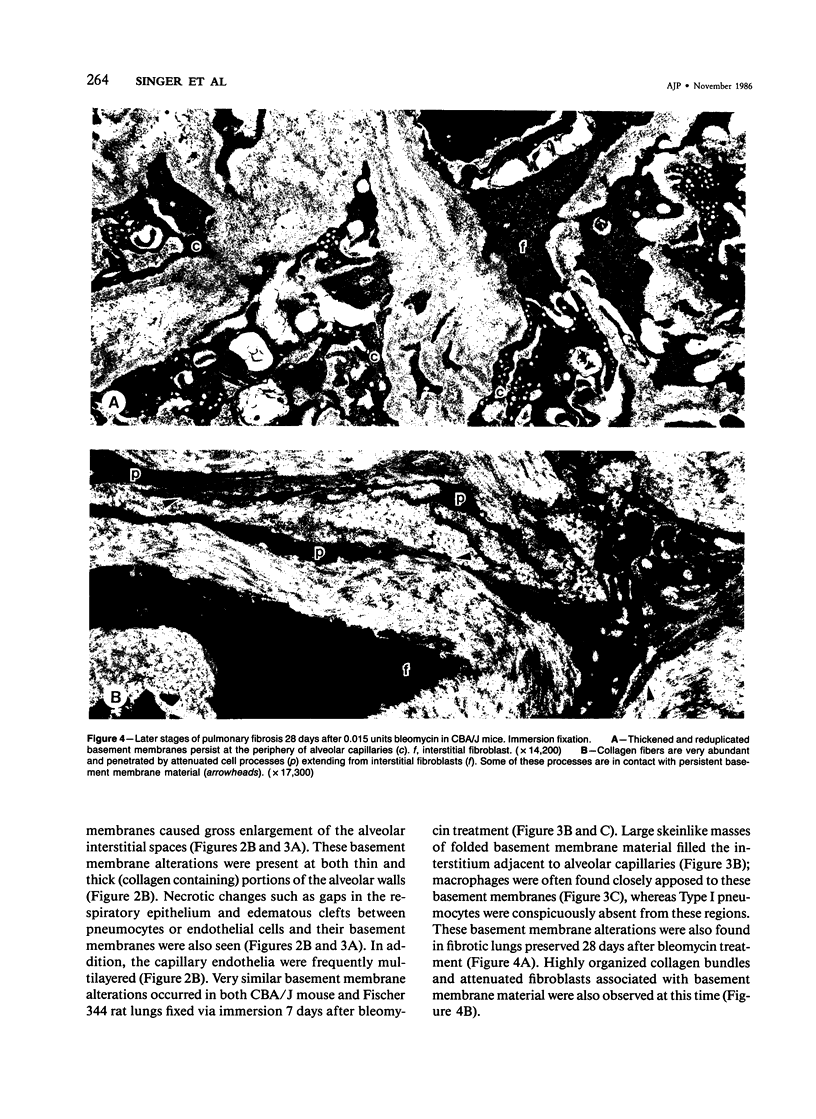
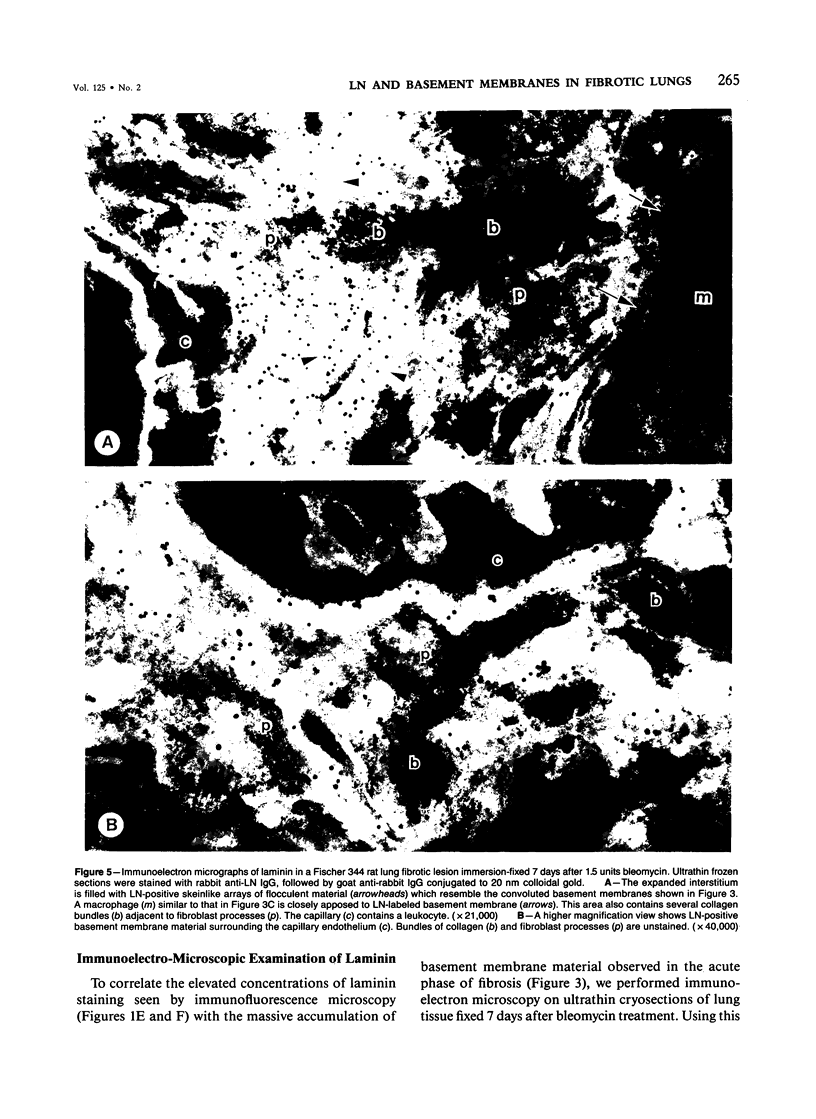
The distribution of laminin was studied during pulmonary fibrosis induced in rodents by bleomycin sulfate. Large accumulations of laminin associated with basement membranes were seen in thickened lung interstitial spaces by immunofluorescence microscopy, starting at 7 days (32-75% increases) and persisting through 28 days (66-79% increase). By electron microscopy, these laminin concentrations were skeinlike masses of reduplicated basement membranes localized at the surface of alveolar capillary endothelial cells. Numerous macrophages were also associated with this basement membrane material. These findings suggest that bleomycin-induced damage to lung cells causes massive local accumulations of basement membranes, which might be involved in the expansion of the interstitial stroma by stimulating attachment and activation of certain inflammatory cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pathol. 1983 Aug;112(2):224–230. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974 Nov;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y. Drug-induced pulmonary fibrosis. Environ Health Perspect. 1984 Apr;55:25–36. doi: 10.1289/ehp.845525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Bradley K., McConnell-Breul S., Crystal R. G. Lung collagen heterogeneity. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2828–2832. doi: 10.1073/pnas.71.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J. O., Osman M., Cerreta J. M., Mandl I., Turino G. M. Glycosaminoglycan synthesis in explants derived from bleomycin-treated fibrotic hamster lungs. Proc Soc Exp Biol Med. 1983 Jul;173(3):362–366. doi: 10.3181/00379727-173-41657. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Overton J. E., Marino B. A., Uitto J., Starcher B. C. Collagen biosynthesis in bleomycin-induced pulmonary fibrosis in hamsters. J Lab Clin Med. 1980 Dec;96(6):943–953. [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Leukocyte chemotaxis. Fed Proc. 1983 Aug;42(11):2851–2862. [PubMed] [Google Scholar]
- Gil J., Martinez-Hernandez A. The connective tissue of the rat lung: electron immunohistochemical studies. J Histochem Cytochem. 1984 Feb;32(2):230–238. doi: 10.1177/32.2.6363520. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Bradley K., Crystal R. G. Lung collagen heterogeneity. Synthesis of type I and type III collagen by rabbit and human lung cells in culture. J Clin Invest. 1976 Jan;57(1):102–111. doi: 10.1172/JCI108250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Tokuyasu K. T., Dutton A. H., Singer S. J. An improved procedure for immunoelectron microscopy: ultrathin plastic embedding of immunolabeled ultrathin frozen sections. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5744–5747. doi: 10.1073/pnas.81.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick P., d'Ardenne A. J. Effects of fixation and enzymatic digestion on the immunohistochemical demonstration of laminin and fibronectin in paraffin embedded tissue. J Clin Pathol. 1984 Jun;37(6):639–644. doi: 10.1136/jcp.37.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler W. Formal genesis of pulmonary fibrosis: experimental investigations. Curr Top Pathol. 1983;73:207–231. doi: 10.1007/978-3-642-69134-8_5. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Humphreys C. J. Lack of metabolism as the biochemical basis of bleomycin-induced pulmonary toxicity. Proc Natl Acad Sci U S A. 1983 May;80(10):3064–3068. doi: 10.1073/pnas.80.10.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna M. A., Bedrossian C. W., Lichtiger B., Salem P. A. Interstitial pneumonitis associated with bleomycin therapy. Am J Clin Pathol. 1972 Nov;58(5):501–510. doi: 10.1093/ajcp/58.5.501. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Bano M., Kidwell W. R., Oppenheim J. J. Interleukin 1 increases collagen type IV production by murine mammary epithelial cells. J Immunol. 1985 Feb;134(2):904–909. [PubMed] [Google Scholar]
- McArdle J. P., Müller H. K., Roff B. T., Murphy W. H. Basal lamina redevelopment in tumours metastatic to brain:an immunoperoxidase study using an antibody to type-IV collagen. Int J Cancer. 1984 Nov 15;34(5):633–638. doi: 10.1002/ijc.2910340508. [DOI] [PubMed] [Google Scholar]
- Montesano R., Mossaz A., Ryser J. E., Orci L., Vassalli P. Leukocyte interleukins induce cultured endothelial cells to produce a highly organized, glycosaminoglycan-rich pericellular matrix. J Cell Biol. 1984 Nov;99(5):1706–1715. doi: 10.1083/jcb.99.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., Thrall R. S., Ward P. A. Bleomycin-induced pulmonary fibrosis in rats: biochemical demonstration of increased rate of collagen synthesis. Am Rev Respir Dis. 1980 Mar;121(3):501–506. doi: 10.1164/arrd.1980.121.3.501. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Nakane P. K. Basement membranes. Synthesis and deposition in response to cellular injury. Lab Invest. 1969 Jul;21(1):27–41. [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer D., Giordana M. T., Mauro A., Migheli A. GFAP, F VIII/RAg, laminin, and fibronectin in gliosarcomas: an immunohistochemical study. Acta Neuropathol. 1984;63(2):108–116. doi: 10.1007/BF00697192. [DOI] [PubMed] [Google Scholar]
- Schoenberger C. I., Rennard S. I., Bitterman P. B., Fukuda Y., Ferrans V. J., Crystal R. G. Paraquat-induced pulmonary fibrosis. Role of the alveolitis in modulating the development of fibrosis. Am Rev Respir Dis. 1984 Jan;129(1):168–173. doi: 10.1164/arrd.1984.129.1.168. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Kunkel R. G., Phan S. H. The role of strain variation in murine bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1983 Jan;127(1):63–66. doi: 10.1164/arrd.1983.127.1.63. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H., McGarry B. M. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983 May;127(5):614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H. Modulation of bleomycin-induced pulmonary fibrosis in the BALB/c mouse by cyclophosphamide-sensitive T cells. Am J Pathol. 1984 Aug;116(2):270–278. [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Salmon M., Morris C. J., Wainwright A. C., Walton K. W. Laminin and vascular proliferation in rheumatoid arthritis. Ann Rheum Dis. 1984 Aug;43(4):551–555. doi: 10.1136/ard.43.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider G. L., Hayes J. A., Korthy A. L. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: pathology and stereology. Am Rev Respir Dis. 1978 Jun;117(6):1099–1108. doi: 10.1164/arrd.1978.117.6.1099. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Thorning D., Vracko R. Renal glomerular basal lamina scaffold: embryologic development, anatomy, and role in cellular reconstruction of rat glomeruli injured by freezing and thawing. Lab Invest. 1977 Jul;37(1):105–119. [PubMed] [Google Scholar]
- Thrall R. S., McCormick J. R., Jack R. M., McReynolds R. A., Ward P. A. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979 Apr;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Torikata C., Villiger B., Kuhn C., 3rd, McDonald J. A. Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab Invest. 1985 Apr;52(4):399–408. [PubMed] [Google Scholar]
- Vaccaro C. A., Brody J. S., Snider G. L. Alveolar wall basement membranes in bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1985 Oct;132(4):905–912. doi: 10.1164/arrd.1985.132.4.905. [DOI] [PubMed] [Google Scholar]
- Vaccaro C. A., Brody J. S. Ultrastructural localization and characterization of proteoglycans in the pulmonary alveolus. Am Rev Respir Dis. 1979 Oct;120(4):901–910. doi: 10.1164/arrd.1979.120.4.901. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Huard T. K. Macrophages express cell surface laminin. Exp Cell Res. 1983 Feb;143(2):475–479. doi: 10.1016/0014-4827(83)90077-0. [DOI] [PubMed] [Google Scholar]