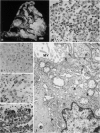
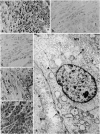
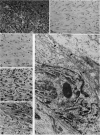
Abstract
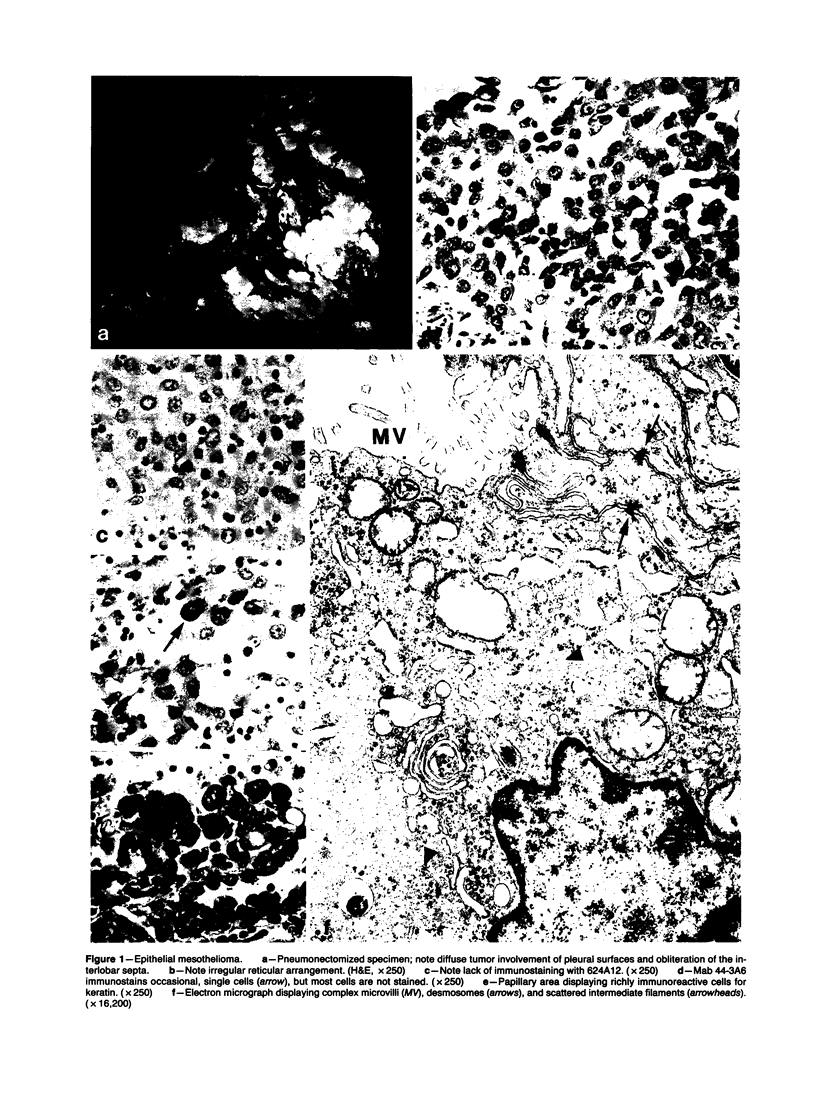
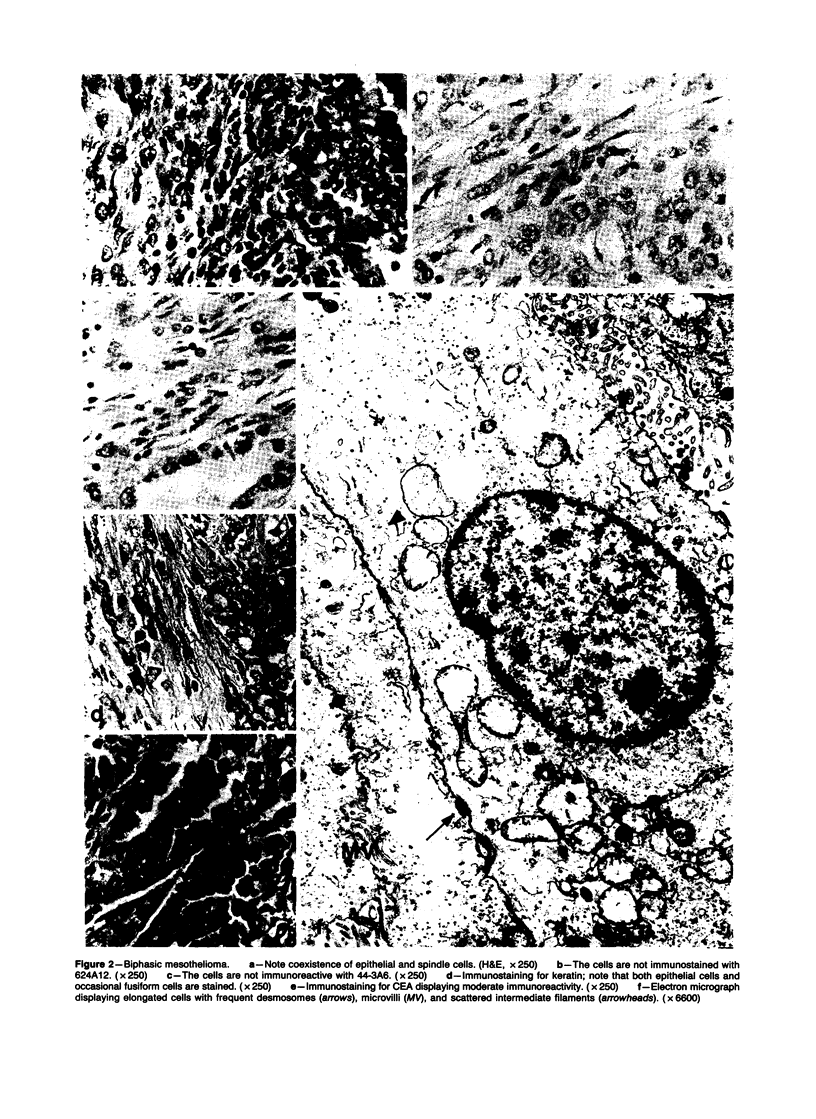
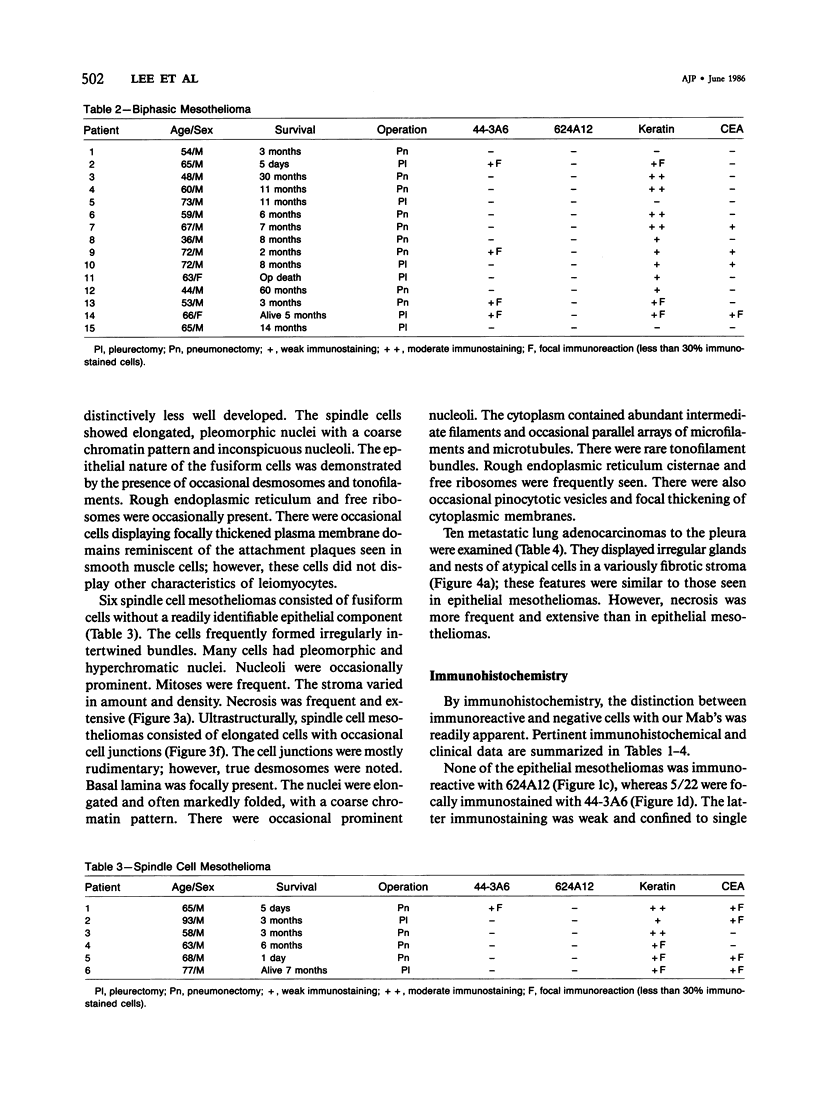
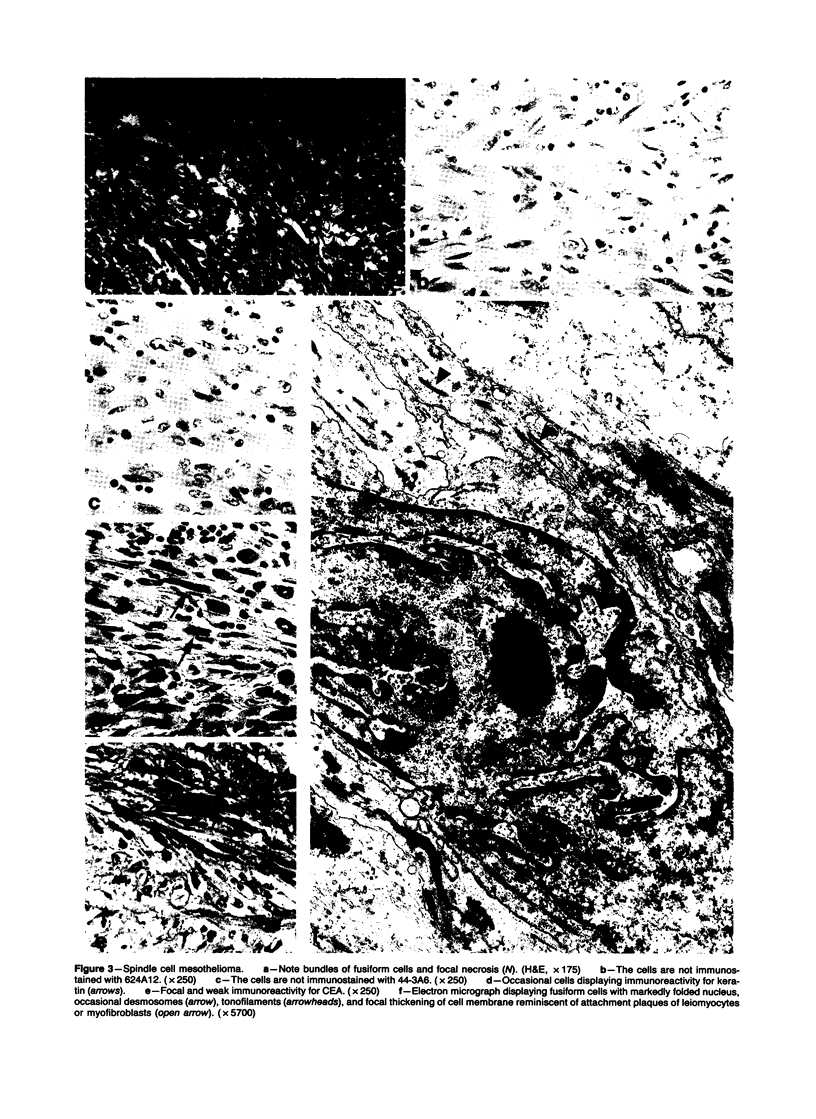
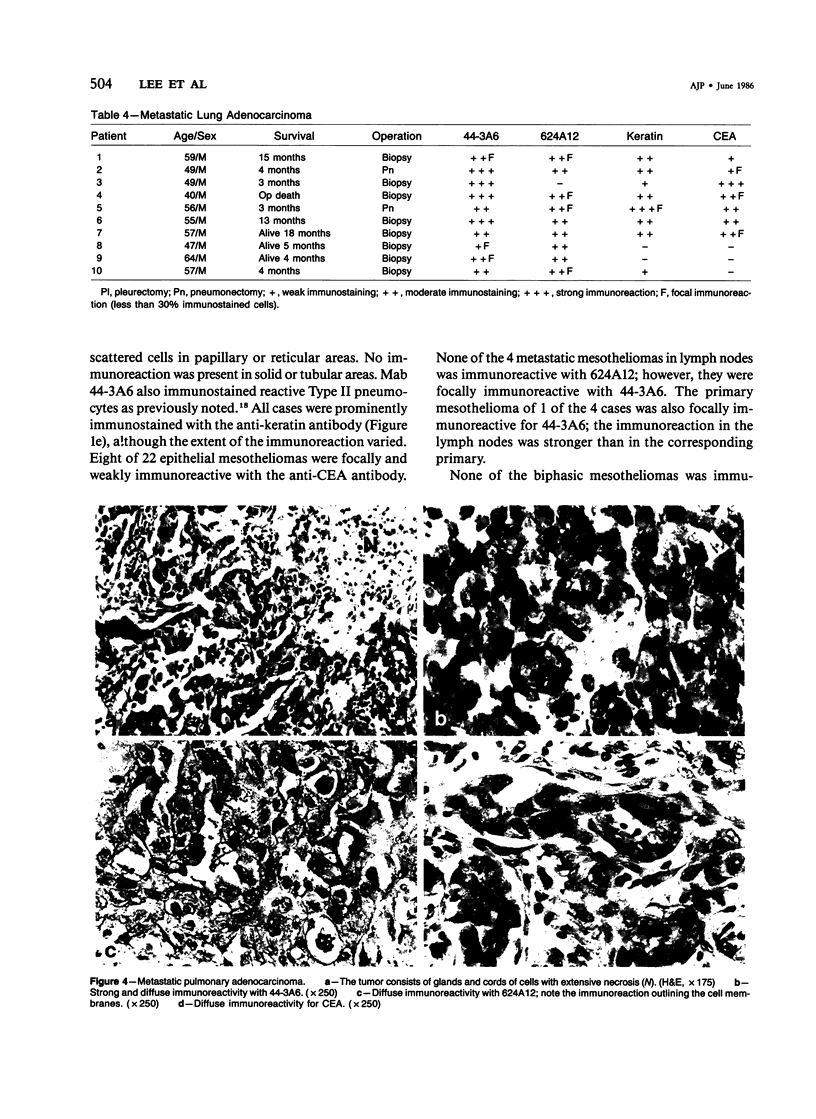
Forty-three malignant pleural mesotheliomas and 10 known metastatic pulmonary adenocarcinomas to the pleura were studied by immunohistochemistry using monoclonal antibodies 44-3A6 and 624A12. Monoclonal antibodies 44-3A6 and 624A12 were raised against human pulmonary carcinoma cell lines; they recognize a membrane-associated protein of 40,000 mol wt and a specific sugar sequence of lacto-N-fucopentose III, respectively. Samples were also studied with a broad-spectrum antikeratin antibody and a polyclonal antibody to carcinoembryonic antigen (CEA). These investigations were performed on formalin-fixed and paraffin-embedded tissues. The mesotheliomas comprised only grossly evident, pleurectomized, or pneumonectomized cases; they included 22 epithelial, 15 biphasic, and 6 spindle cell types. Electron-microscopic study was also done on 9 cases. None of the mesotheliomas was immunoreactive to 624A12, while 9/10 metastatic pulmonary adenocarcinomas were convincingly immunoreactive. Monoclonal antibody 44-3A6 immunostained all of the metastatic adenocarcinomas strongly, whereas only 10/43 mesotheliomas were focally and weakly immunoreactive. The latter included 5 epithelial and 4 biophasic mesotheliomas and 1 spindle cell mesotheliomas; the immunoreaction was confined to scattered single cells, and the staining pattern was readily discernible from that of adenocarcinomas. Forty of 43 mesotheliomas were strongly immunoreactive with the broad-spectrum anti-keratin antibody, whereas 8/10 metastatic pulmonary adenocarcinomas showed focal and rather weak staining. Seven of 10 metastatic adenocarcinomas were immunoreactive to anti-CEA antibody, while only 15/43 mesotheliomas displayed weak immunoreactivity. It is concluded that monoclonal antibodies 44-3A6 and 624A12 are excellent phenotypic markers of metastatic pulmonary adenocarcinomas to the pleura and thus are useful for the differential diagnosis of pleural mesotheliomas. Given conventionally fixed and processed tissues, it appears that the combined use of these monoclonal antibodies may be more effective for that differential diagnosis than anti-CEA and anti-keratin antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner B. F., Gould V. E., Radosevich J. A., Ma Y., Lee I., Rosen S. T. Application of monoclonal antibody 44-3A6 in the cytodiagnosis and classification of pulmonary carcinomas. Diagn Cytopathol. 1985 Oct-Dec;1(4):300–307. doi: 10.1002/dc.2840010408. [DOI] [PubMed] [Google Scholar]
- Battifora H., Kopinski M. I. Distinction of mesothelioma from adenocarcinoma. An immunohistochemical approach. Cancer. 1985 Apr 15;55(8):1679–1685. doi: 10.1002/1097-0142(19850415)55:8<1679::aid-cncr2820550812>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Blobel G. A., Gould V. E., Moll R., Lee I., Huszar M., Geiger B., Franke W. W. Coexpression of neuroendocrine markers and epithelial cytoskeletal proteins in bronchopulmonary neuroendocrine neoplasms. Lab Invest. 1985 Jan;52(1):39–51. [PubMed] [Google Scholar]
- Blobel G. A., Moll R., Franke W. W., Kayser K. W., Gould V. E. The intermediate filament cytoskeleton of malignant mesotheliomas and its diagnostic significance. Am J Pathol. 1985 Nov;121(2):235–247. [PMC free article] [PubMed] [Google Scholar]
- Blobel G. A., Moll R., Franke W. W., Vogt-Moykopf I. Cytokeratins in normal lung and lung carcinomas. I. Adenocarcinomas, squamous cell carcinomas and cultured cell lines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):407–429. doi: 10.1007/BF02889883. [DOI] [PubMed] [Google Scholar]
- Bolen J. W., Thorning D. Mesotheliomas: a light- and electron-microscopical study concerning histogenetic relationships between the epithelial and the mesenchymal variants. Am J Surg Pathol. 1980 Oct;4(5):451–464. [PubMed] [Google Scholar]
- Chiu B., Churg A., Tengblad A., Pearce R., McCaughey W. T. Analysis of hyaluronic acid in the diagnosis of malignant mesothelioma. Cancer. 1984 Nov 15;54(10):2195–2199. doi: 10.1002/1097-0142(19841115)54:10<2195::aid-cncr2820541021>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Henkart P. A., Todd C. W., Terry W. D. Heterogeneity of the carcinoembryonic antigen. Immunochemistry. 1973 Sep;10(9):591–599. doi: 10.1016/0019-2791(73)90160-2. [DOI] [PubMed] [Google Scholar]
- Combs S. G., Marder R. J., Minna J. D., Mulshine J. L., Polovina M. R., Rosen S. T. Immunohistochemical localization of the immunodominant differentiation antigen lacto-N-fucopentaose III in normal adult and fetal tissues. J Histochem Cytochem. 1984 Sep;32(9):982–988. doi: 10.1177/32.9.6379043. [DOI] [PubMed] [Google Scholar]
- Corson J. M., Pinkus G. S. Mesothelioma: profile of keratin proteins and carcinoembryonic antigen: an immunoperoxidase study of 20 cases and comparison with pulmonary adenocarcinomas. Am J Pathol. 1982 Jul;108(1):80–88. [PMC free article] [PubMed] [Google Scholar]
- GOLD P., FREEDMAN S. O. DEMONSTRATION OF TUMOR-SPECIFIC ANTIGENS IN HUMAN COLONIC CARCINOMATA BY IMMUNOLOGICAL TOLERANCE AND ABSORPTION TECHNIQUES. J Exp Med. 1965 Mar 1;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Russell E. K., Sims H. L., Baylin S. B., Bunn P. A., Jr, Guccion J. G., Minna J. D. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980 Oct;40(10):3502–3507. [PubMed] [Google Scholar]
- Grunert F., Luckenbach G. A., Haderlie B., Schwarz K., von Kleist S. Comparison of colon-, lung-, and breast-derived carcinoembryonic antigen and cross-reacting antigens by monoclonal antibodies and fingerprint analysis. Ann N Y Acad Sci. 1983;417:75–85. doi: 10.1111/j.1749-6632.1983.tb32851.x. [DOI] [PubMed] [Google Scholar]
- Grunert F., Wank K., Luckenbach G. A., Von Kleist S. Monoclonal antibodies against CEA. Comparison of the immunoprecipitates by fingerprint analysis. Oncodev Biol Med. 1982;3(2-3):191–200. [PubMed] [Google Scholar]
- Holden J., Churg A. Immunohistochemical staining for keratin and carcinoembryonic antigen in the diagnosis of malignant mesothelioma. Am J Surg Pathol. 1984 Apr;8(4):277–279. doi: 10.1097/00000478-198404000-00004. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huang L. C., Brockhaus M., Magnani J. L., Cuttitta F., Rosen S., Minna J. D., Ginsburg V. Many monoclonal antibodies with an apparent specificity for certain lung cancers are directed against a sugar sequence found in lacto-N-fucopentaose III. Arch Biochem Biophys. 1983 Jan;220(1):318–320. doi: 10.1016/0003-9861(83)90417-4. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Lee I., Radosevich J. A., Ma Y. X., Combs S. G., Rosen S. T., Gould V. E. Immunohistochemical analysis of human pulmonary carcinomas using monoclonal antibody 44-3A6. Cancer Res. 1985 Nov;45(11 Pt 2):5813–5817. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Krepler R., Franke W. W. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Nap M., Keuning H., Burtin P., Oosterhuis J. W., Fleuren G. CEA and NCA in benign and malignant breast tumors. Am J Clin Pathol. 1984 Nov;82(5):526–534. doi: 10.1093/ajcp/82.5.526. [DOI] [PubMed] [Google Scholar]
- Nap M., ten Hoor K. A., Fleuren G. J. Cross-reactivity with normal antigens in commercial anti-CEA sera, used for immunohistology. The need for tissue controls and absorptions. Am J Clin Pathol. 1983 Jan;79(1):25–31. doi: 10.1093/ajcp/79.1.25. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pascal R. R., Mesa-Tejada R., Bennett S. J., Garces A., Fenoglio C. M. Carcinoembryonic antigen: immunohistologic identification in invasive and intraepithelial carcinomas of the lung. Arch Pathol Lab Med. 1977 Nov;101(11):568–571. [PubMed] [Google Scholar]
- Radosevich J. A., Ma Y. X., Lee I., Salwen H. R., Gould V. E., Rosen S. T. Monoclonal antibody 44-3A6 as a probe for a novel antigen found on human lung carcinomas with glandular differentiation. Cancer Res. 1985 Nov;45(11 Pt 2):5808–5812. [PubMed] [Google Scholar]
- Rosen S. T., Mulshine J. L., Cuttitta F., Fedorko J., Carney D. N., Gazdar A. F., Minna J. D. Analysis of human small cell lung cancer differentiation antigens using a panel of rat monoclonal antibodies. Cancer Res. 1984 May;44(5):2052–2061. [PubMed] [Google Scholar]
- Said J. W., Nash G., Tepper G., Banks-Schlegel S. Keratin proteins and carcinoembryonic antigen in lung carcinoma: an immunoperoxidase study of fifty-four cases, with ultrastructural correlations. Hum Pathol. 1983 Jan;14(1):70–76. doi: 10.1016/s0046-8177(83)80048-3. [DOI] [PubMed] [Google Scholar]
- Singh G., Whiteside T. L., Dekker A. Immunodiagnosis of mesothelioma: use of antimesothelial cell serum in an indirect immunofluorescence assay. Cancer. 1979 Jun;43(6):2288–2296. doi: 10.1002/1097-0142(197906)43:6<2288::aid-cncr2820430619>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Walts A. E., Said J. W., Shintaku I. P., Sassoon A. F., Banks-Schlegel S. Keratins of different molecular weight in exfoliated mesothelial and adenocarcinoma cells--an aid to cell identification. Am J Clin Pathol. 1984 Apr;81(4):442–446. doi: 10.1093/ajcp/81.4.442. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Huang S. N., Gold P. Absence of carcinoembryonic antigen-like material in mesothelioma: an immunohistochemical differentiation from other lung cancers. Cancer. 1979 Sep;44(3):937–943. doi: 10.1002/1097-0142(197909)44:3<937::aid-cncr2820440322>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Warhol M. J., Hickey W. F., Corson J. M. Malignant mesothelioma: ultrastructural distinction from adenocarcinoma. Am J Surg Pathol. 1982 Jun;6(4):307–314. [PubMed] [Google Scholar]