Abstract
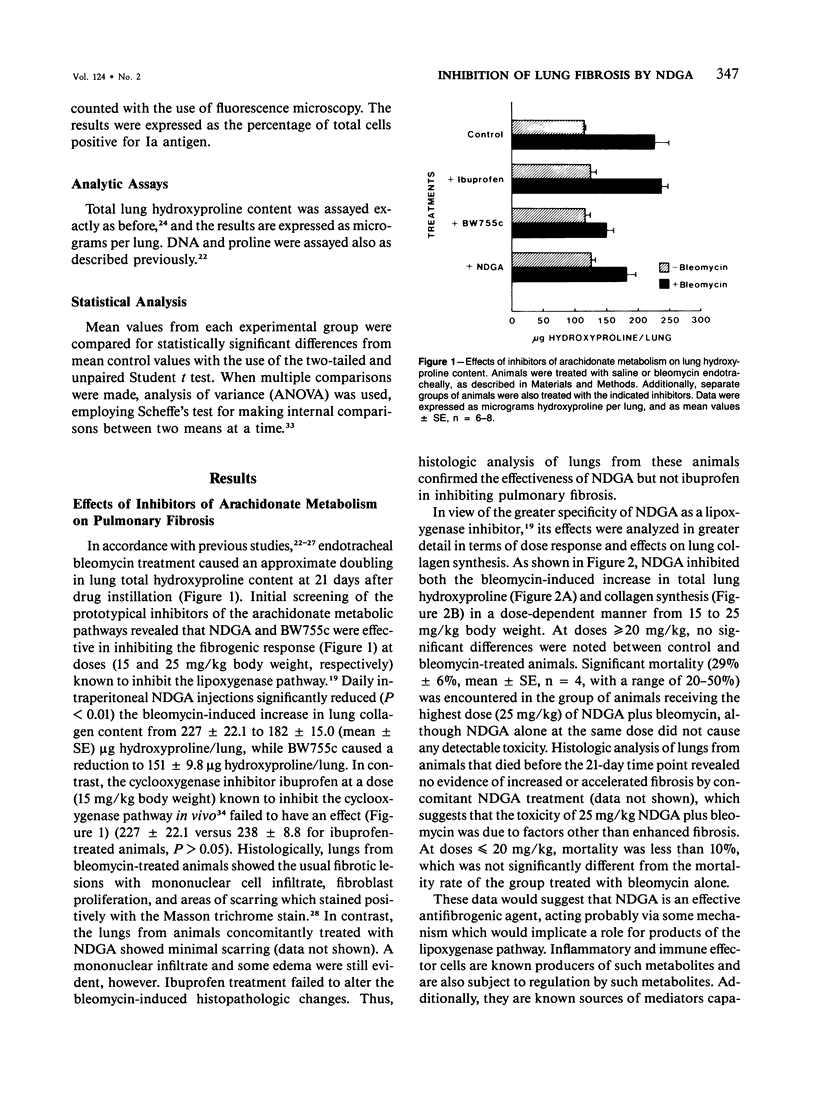
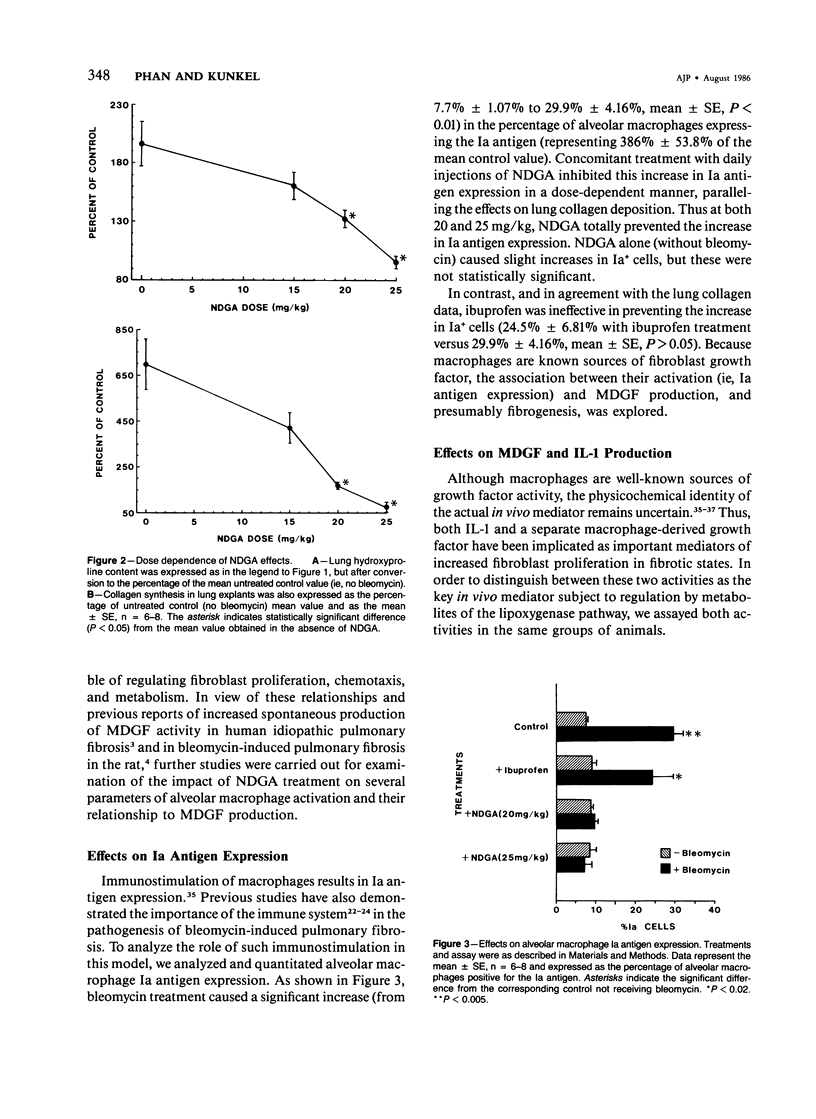
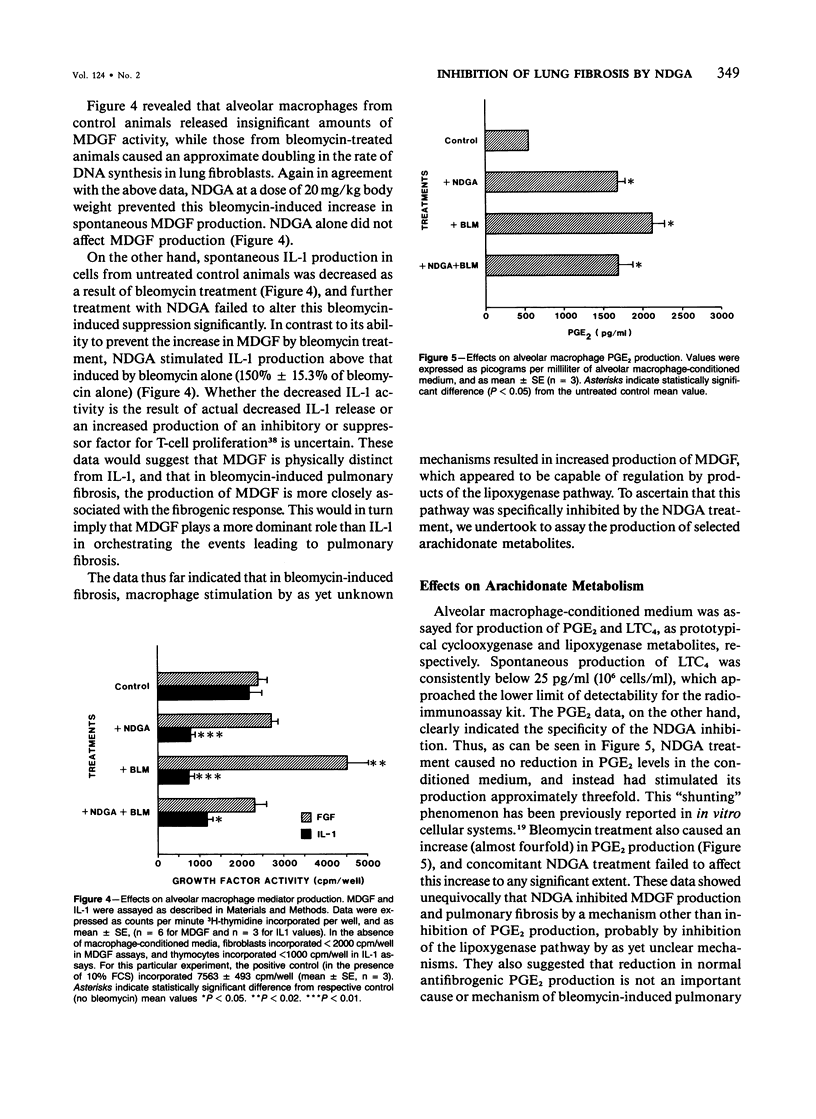
The role of alveolar macrophage activation and release of mediators remains unclear. In this study, this role is examined with respect to the effects of relatively selective inhibitors of arachidonate metabolism on the pathogenesis of pulmonary fibrosis. CBA/J mice were administered bleomycin (0.037 units) endotracheally to induce pulmonary fibrosis. Daily intraperitoneal injections of a lipoxygenase inhibitor, nordihydroguaiaretic acid (NDGA) inhibited pulmonary fibrosis in a dose-dependent manner (15-25 mg/kg body weight), as assessed by both lung collagen synthesis and total lung hydroxyproline content. The less specific inhibitor BW755c was also effective at a dose of 25 mg/kg. In contrast, the cyclooxygenase inhibitor, ibuprofen (15 mg/kg), was completely ineffective. Correlated with this antifibrogenic activity of NDGA was the inhibition of several other parameters of bleomycin-induced pulmonary fibrosis. Bleomycin treatment caused a greater than threefold increase in the percentage of alveolar macrophages expressing Ia antigen (from 7.7% +/- 1.07% to 29.9% +/- 4.16% of total recoverable alveolar macrophages). NDGA, but not ibuprofen, inhibited this increase in a dose-dependent manner. Associated with this indication of macrophage stimulation was an increase in spontaneous macrophage production of fibroblast growth factor (MDGF) activity as a result of bleomycin instillation. This increase was also inhibited by NDGA treatment. In contrast, bleomycin treatment caused a reduction in alveolar macrophage interleukin-1 (IL-1) production, and NDGA treatment did not alter this reduction, which suggests that MDGF is separate from IL-1 in this case, and that MDGF played a more dominant role, at least in this model of pulmonary fibrosis. This antifibrogenic activity of NDGA was accomplished without any reduction in spontaneous macrophage prostaglandin (PG)E2 production, which suggests the selectivity (versus cyclooxygenase pathway) of NDGA inhibition and the relative lack of importance of macrophage-derived PGE2 in modulating fibrogenesis in this model. The results of this study have thus demonstrated the importance of alveolar macrophage stimulation and increased production of MDGF in the pathogenesis of bleomycin-induced pulmonary fibrosis. The data also suggest that both macrophage parameters are subject to regulation by arachidonate metabolites.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Bryant R. W., Low C. E., Pupillo M. B., Vanderhoek J. Y. Regulation of T-lymphocyte mitogenesis by the leukocyte product 15-hydroxy-eicosatetraenoic acid (15-HETE). Cell Immunol. 1982 Feb;67(1):112–120. doi: 10.1016/0008-8749(82)90203-9. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Chandler D. B., Giri S. N., Chen Z., Hyde D. M. The in vitro synthesis and degradation of prostaglandins during the development of bleomycin-induced pulmonary fibrosis in hamsters. Prostaglandins Leukot Med. 1983 May;11(1):11–31. doi: 10.1016/0262-1746(83)90105-1. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Kunkel S. L., Ward P. A., Higashi G. I. Exogenously administered prostaglandins modulate pulmonary granulomas induced by Schistosoma mansoni eggs. Am J Pathol. 1983 Apr;111(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Bleomycin-induced pulmonary fibrosis in hamsters. An alveolar macrophage product increases fibroblast prostaglandin E2 and cyclic adenosine monophosphate and suppresses fibroblast proliferation and collagen production. J Clin Invest. 1983 Dec;72(6):2082–2091. doi: 10.1172/JCI111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Kuhn C., 3rd Bleomycin-induced pulmonary fibrosis in hamsters: effect of neutrophil depletion on lung collagen synthesis. Am Rev Respir Dis. 1982 Oct;126(4):737–739. doi: 10.1164/arrd.1982.126.4.737. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Marnoy S. O., Rosenwasser L. J. Role of arachidonate metabolism in the immunoregulatory function of human leukocytic pyrogen/lymphocyte-activating factor/interleukin 1. J Immunol. 1983 Feb;130(2):890–895. [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985 Aug 1;162(2):516–527. doi: 10.1084/jem.162.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Zurier R. B., Schreiber A. D., Leff J. A., Daniele R. P. Monocyte inhibition of lung fibroblast growth: relationship to fibroblast prostaglandin production and density-defined monocyte subpopulations. J Leukoc Biol. 1985 Jan;37(1):15–28. doi: 10.1002/jlb.37.1.15. [DOI] [PubMed] [Google Scholar]
- Estes J. E., Pledger W. J., Gillespie G. Y. Macrophage-derived growth factor for fibroblasts and Interleukin-1 are distinct entities. J Leukoc Biol. 1984 Jan;35(1):115–129. doi: 10.1002/jlb.35.1.115. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Bundy G. L. Hapten mimic elicits antibodies recognizing prostaglandin E2. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2689–2693. doi: 10.1073/pnas.75.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan L., Huang C. H., Prestayko A. W., Stout J. T., Evans J. E., Crooke S. T. Inhibition of bleomycin-induced DNA breakage by superoxide dismutase. Cancer Res. 1981 Dec;41(12 Pt 1):5103–5106. [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Lands W. E. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem. 1980 Jul 10;255(13):6253–6261. [PubMed] [Google Scholar]
- Kimberly R. P., Bowden R. E., Keiser H. R., Plotz P. H. Reduction of renal function by newer nonsteroidal anti-inflammatory drugs. Am J Med. 1978 May;64(5):804–807. doi: 10.1016/0002-9343(78)90520-x. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Secretion of macrophage-derived growth factor during acute lung injury induced by bleomycin. J Leukoc Biol. 1985 Jan;37(1):1–14. doi: 10.1002/jlb.37.1.1. [DOI] [PubMed] [Google Scholar]
- Krakauer T. A macrophage-derived factor that inhibits the production and action of interleukin 2. J Leukoc Biol. 1985 Sep;38(3):429–439. doi: 10.1002/jlb.38.3.429. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Mouton C., Higashi G. I. Role of lipoxygenase products in murine pulmonary granuloma formation. J Clin Invest. 1984 Aug;74(2):514–524. doi: 10.1172/JCI111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Thrall R. S., Kunkel R. G., McCormick J. R., Ward P. A., Zurier R. B. Suppression of immune complex vasculitis in rats by prostaglandin. J Clin Invest. 1979 Nov;64(5):1525–1529. doi: 10.1172/JCI109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Mensing H., Czarnetzki B. M. Leukotriene B4 induces in vitro fibroblast chemotaxis. J Invest Dermatol. 1984 Jan;82(1):9–12. doi: 10.1111/1523-1747.ep12258678. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Schubotz L. M., Weser U., Kivirikko K. I. Involvement of superoxide in the prolyl and lysyl hydroxylase reactions. Biochem Biophys Res Commun. 1979 Jul 12;89(1):98–102. doi: 10.1016/0006-291x(79)90948-3. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Thomas M. J., Lees C. J., McCall C. E. Neutrophil-aggregating activity of monohydroxyeicosatetraenoic acids. Am J Pathol. 1981 Jul;104(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Pawlowski N. A., Kaplan G., Hamill A. L., Cohn Z. A., Scott W. A. Arachidonic acid metabolism by human monocytes. Studies with platelet-depleted cultures. J Exp Med. 1983 Aug 1;158(2):393–412. doi: 10.1084/jem.158.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin J. M., Langner R. O. Effects of dimethyl sulfoxide (DMSO) on bleomycin-induced pulmonary fibrosis. Biochem Pharmacol. 1985 Jul 1;34(13):2386–2389. doi: 10.1016/0006-2952(85)90799-3. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Thrall R. S., Williams C. Bleomycin-induced pulmonary fibrosis. Effects of steroid on lung collagen metabolism. Am Rev Respir Dis. 1981 Oct;124(4):428–434. doi: 10.1164/arrd.1981.124.4.428. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Varani J., Smith D. Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J Clin Invest. 1985 Jul;76(1):241–247. doi: 10.1172/JCI111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Induction of fibroblast proliferation by human mononuclear leukocyte-derived proteins. Arthritis Rheum. 1983 Jan;26(1):22–27. doi: 10.1002/art.1780260104. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S. A., Mitchell M. D. Arachidonate lipoxygenase activity in human fetal lung. Eur J Pharmacol. 1982 Mar 12;78(3):389–391. doi: 10.1016/0014-2999(82)90047-4. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H., McGarry B. M. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983 May;127(5):614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H. Modulation of bleomycin-induced pulmonary fibrosis in the BALB/c mouse by cyclophosphamide-sensitive T cells. Am J Pathol. 1984 Aug;116(2):270–278. [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y. The production of hydroxyl radical from copper(I) complex systems of bleomycin and tallysomycin: comparison with copper(II) and iron(II) systems. Biochem Biophys Res Commun. 1979 Sep 12;90(1):375–383. doi: 10.1016/0006-291x(79)91635-8. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L., LUNDBERG W. O., BOYER P. D. Effect of temperature and antioxidants upon the lipoxidase-catalyzed oxidation of sodium linoleate. Arch Biochem Biophys. 1953 Feb;42(2):293–304. doi: 10.1016/0003-9861(53)90359-2. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W. A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1984 Feb;129(2):279–283. [PubMed] [Google Scholar]
- Thrall R. S., McCormick J. R., Jack R. M., McReynolds R. A., Ward P. A. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979 Apr;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Thrall R. S., Phan S. H., McCormick J. R., Ward P. A. The development of bleomycin-induced pulmonary fibrosis in neutrophil-depleted and complement-depleted rats. Am J Pathol. 1981 Oct;105(1):76–81. [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Ono T., Ogawa T., Kawanishi Y. Pyridinoline is a real moiety of collagen. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1407–1412. doi: 10.1016/0006-291x(82)91406-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y., Helsel W. E., Wahl S. M. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981 Aug;127(2):673–678. [PubMed] [Google Scholar]
- Uotila P., Männistö J., Simberg N., Hartiala K. Indomethacin inhibits arachidonic acid metabolism via lipoxygenase and cyclo-oxygenase in hamster isolated lungs. Prostaglandins Med. 1981 Dec;7(6):591–599. doi: 10.1016/0161-4630(81)90049-5. [DOI] [PubMed] [Google Scholar]
- VanRollins M., Ho S. H., Greenwald J. E., Alexander M., Dorman N. J., Wong L. K., Horrocks L. A. Complete separation by high performance liquid chromatography of metabolites of arachidonic acid from incubation with human and rabbit platelets. Prostaglandins. 1980 Sep;20(3):571–577. doi: 10.1016/0090-6980(80)90044-1. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Wharton W., Gillespie G. Y., Russell S. W., Pledger W. J. Mitogenic activity elaborated by macrophage-like cell lines acts as competence factor(s) for BALB/c 3T3 cells. J Cell Physiol. 1982 Jan;110(1):93–100. doi: 10.1002/jcp.1041100115. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


