Abstract
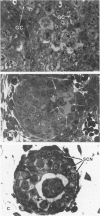
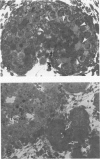
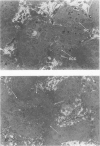
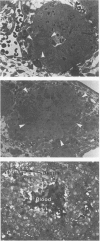
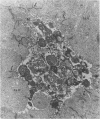
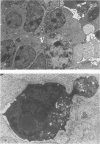
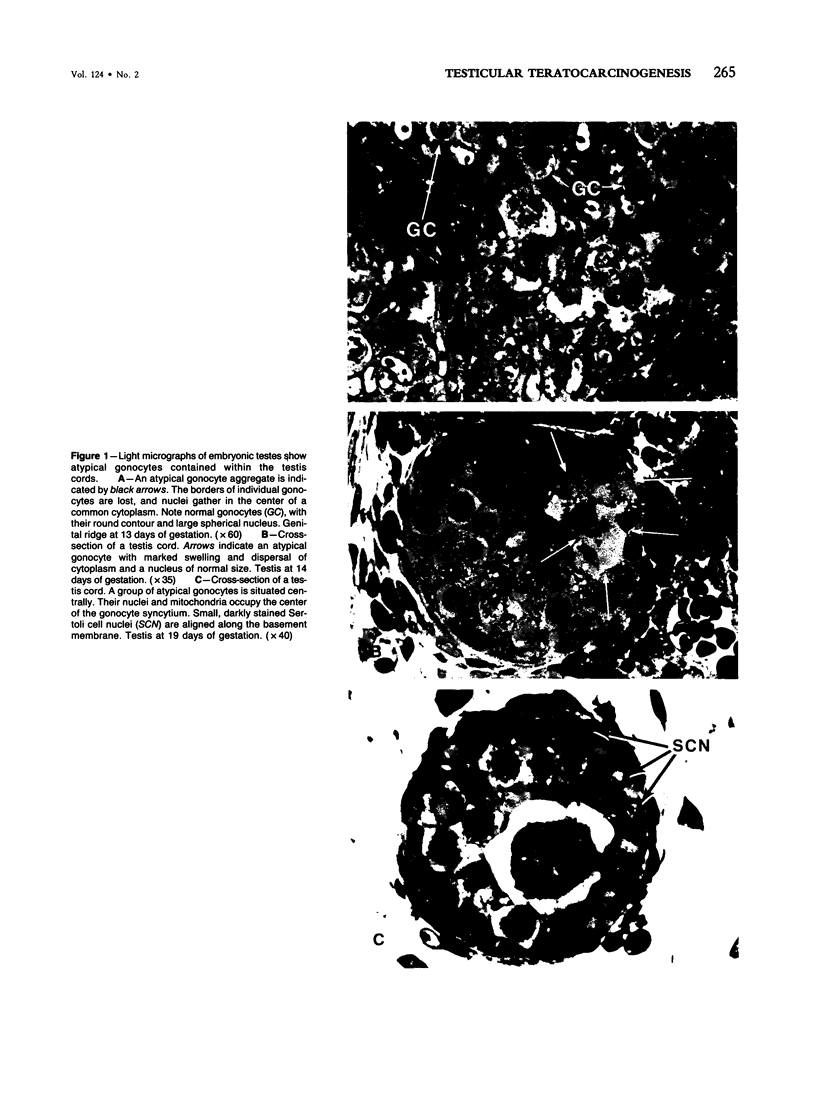
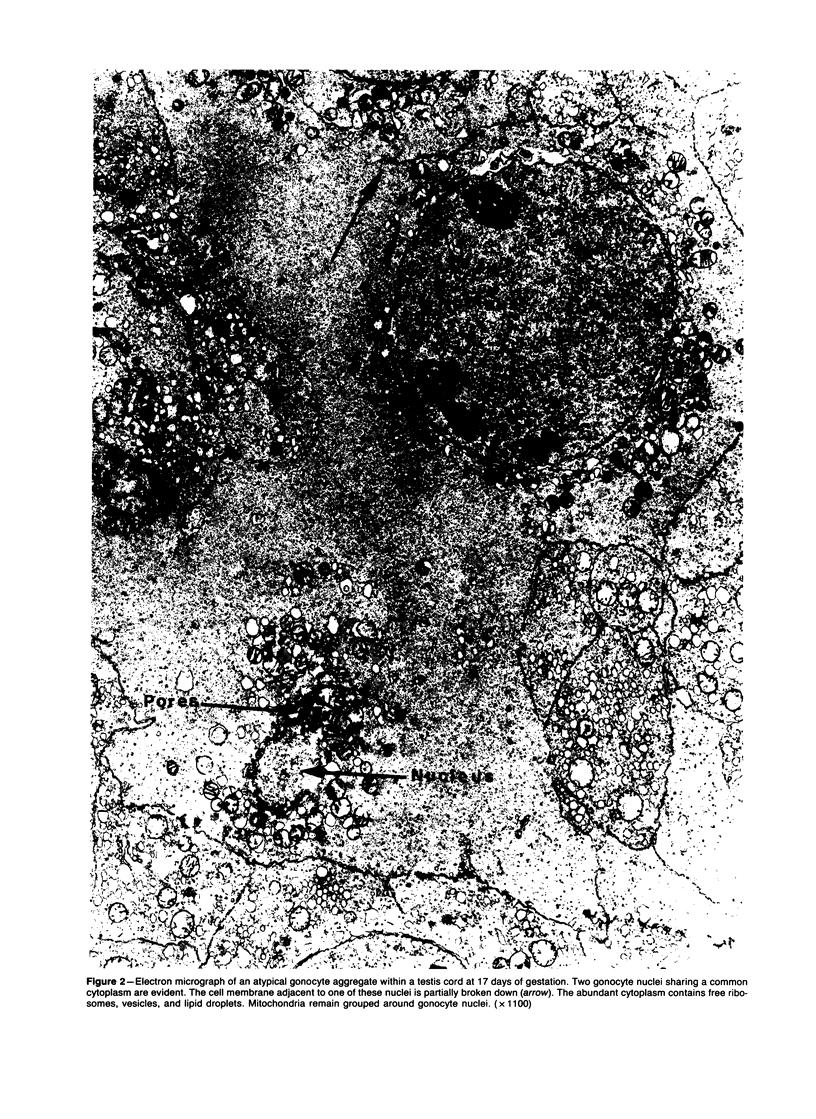
Spontaneous teratocarcinogenesis in the mouse testis begins during the early stages of gonad differentiation. Using inbred strain 129/Sv-ter mice which are highly susceptible to these tumors, the authors have examined the morphologic features of the testis during the gestational period defined from Day 13 through birth. Normal inbred mice (129/J) and random bred mice (Swiss Webster, SW) were used as control groups. Serially sectioned gonads were evaluated at the light- and electron-microscopic levels for histologic changes. In agreement with studies by other workers, embryonal carcinoma cells (ECCs) were observed in tumor-susceptible mice. Cellular arrangements varied from vesicular to nodular. Cell death within advanced tumors was labeled "apoptosis" (shrinkage necrosis). Also encountered were syncytial arrangements of gonocytes (atypical gonocytes), which were present in all animal groups. The significance of atypical gonocytes in relation to degeneration and preneoplasia is addressed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black V. H. Gonocytes in fetal guinea pig testes: phagocytosis of degenerating gonocytes by Sertoli cells. Am J Anat. 1971 Aug;131(4):415–426. doi: 10.1002/aja.1001310403. [DOI] [PubMed] [Google Scholar]
- CARLETON R. L., FRIEDMAN N. B., BOMZE E. J. Experimental teratomas of the testis. Cancer. 1953 May;6(3):464–473. doi: 10.1002/1097-0142(195305)6:3<464::aid-cncr2820060304>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- FRANCHI L. L., MANDL A. M. THE ULTRASTRUCTURE OF GERM CELLS IN FOETAL AND NEONATAL MALE RATS. J Embryol Exp Morphol. 1964 Jun;12:289–308. [PubMed] [Google Scholar]
- Gerstl B., Wong S., Yesner R. Quantitative microscopy of epidermoid lung carcinoma: correlation with survival time. J Natl Cancer Inst. 1976 Mar;56(3):463–469. doi: 10.1093/jnci/56.3.463. [DOI] [PubMed] [Google Scholar]
- Gondos B., Byskov A. G. Germ cell kinetics in the neonatal rabbit testis. Cell Tissue Res. 1981;215(1):143–151. doi: 10.1007/BF00236255. [DOI] [PubMed] [Google Scholar]
- Gondos B., Conner L. A. Ultrastructure of developing germ cells in the fetal rabbit testis. Am J Anat. 1973 Jan;136(1):23–42. doi: 10.1002/aja.1001360104. [DOI] [PubMed] [Google Scholar]
- Gondos B., Hobel C. J. Ultrastructure of germ cell development in the human fetal testis. Z Zellforsch Mikrosk Anat. 1971;119(1):1–20. doi: 10.1007/BF00330535. [DOI] [PubMed] [Google Scholar]
- Gondos B., Renston R. H., Goldstein D. A. Postnatal differentiation of Leydig cells in the rabbit testis. Am J Anat. 1976 Feb;145(2):167–181. doi: 10.1002/aja.1001450203. [DOI] [PubMed] [Google Scholar]
- Gould R. P., Haddad F. Extratubular migration of gonocytes in the foetal rabbit testis. Nature. 1978 Jun 8;273(5662):464–466. doi: 10.1038/273464a0. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Byskov A. G., Christensen I. J., Jensenius J. C. Influence of mesonephros on foetal and neonatal rabbit gonads. I. Sex-steroid release by the testis in vitro. Acta Endocrinol (Copenh) 1982 Feb;99(2):272–280. doi: 10.1530/acta.0.0990272. [DOI] [PubMed] [Google Scholar]
- Hatier R., Grignon G. Ultrastructural study of Sertoli cells in rat seminiferous tubules during intrauterine life and the postnatal period. Anat Embryol (Berl) 1980;160(1):11–27. doi: 10.1007/BF00315646. [DOI] [PubMed] [Google Scholar]
- Holland B., Wardrop C. A. Haemolysis in childhood. Br Med J (Clin Res Ed) 1985 Aug 3;291(6491):297–298. doi: 10.1136/bmj.291.6491.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett M. A. Biology of testicular tumors. Urol Clin North Am. 1977 Oct;4(3):495–507. [PubMed] [Google Scholar]
- Kerr J. F., Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973 Jun 25;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Searle J. The digestion of cellular fragments within phagolysosomes in carcinoma cells. J Pathol. 1972 Sep;108(1):55–58. doi: 10.1002/path.1711080107. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Stevens L. C. Primordial germ cell proliferation in fetal testes in mouse strains with high and low incidences of congenital testicular teratomas. J Natl Cancer Inst. 1982 Oct;69(4):907–913. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, BEALS T. F. THE ULTRASTRUCTURE OF PRIMORDIAL GERMINAL CELLS OF THE FETAL TESTES AND OF EMBRYONAL CARCINOMA CELLS OF MICE. Cancer Res. 1964 Oct;24:1553–1567. [PubMed] [Google Scholar]
- Peters H. Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc Lond B Biol Sci. 1970 Aug 6;259(828):91–101. doi: 10.1098/rstb.1970.0048. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Jr Ultrastructure of human testicular tumors. Cancer. 1966 Dec;19(12):1963–1983. doi: 10.1002/1097-0142(196612)19:12<1963::aid-cncr2820191224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Russo J., De Rosas J. C. Differentiation of the Leydig cell of the mouse testis during the fetal period--an ultrastructural study. Am J Anat. 1971 Apr;130(4):461–480. doi: 10.1002/aja.1001300407. [DOI] [PubMed] [Google Scholar]
- SAURAMO H. Development, occurrence, function and pathology of the rete ovarii. Acta Obstet Gynecol Scand Suppl. 1954;33(2):29–46. [PubMed] [Google Scholar]
- SMITH A. G., POWELL L. Genesis of teratomas of the testis: a study of normal and zinc-injected testes of roosters. Am J Pathol. 1957 Jul-Aug;33(4):653–669. [PMC free article] [PubMed] [Google Scholar]
- STEVENS L. C. EXPERIMENTAL PRODUCTION OF TESTICULAR TERATOMAS IN MICE. Proc Natl Acad Sci U S A. 1964 Sep;52:654–661. doi: 10.1073/pnas.52.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS L. C. Embryology of testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1959 Dec;23:1249–1295. [PubMed] [Google Scholar]
- STEVENS L. C. Testicular teratomas in fetal mice. J Natl Cancer Inst. 1962 Feb;28:247–267. [PubMed] [Google Scholar]
- Sandow B. A., West N. B., Norman R. L., Brenner R. M. Hormonal control of apoptosis in hamster uterine luminal epithelium. Am J Anat. 1979 Sep;156(1):15–35. doi: 10.1002/aja.1001560103. [DOI] [PubMed] [Google Scholar]
- Searle J., Collins D. J., Harmon B., Kerr J. F. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973 Apr;5(2):163–169. doi: 10.3109/00313027309060831. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Sheldon S., Speers W. C., Lehman J. M. Nucleolar persistence in embryonal carcinoma cells. Exp Cell Res. 1981 Mar;132(1):185–192. doi: 10.1016/0014-4827(81)90094-x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E. Abnormal morphology of germ cells in two infertile men. Acta Pathol Microbiol Scand A. 1972;80(3):374–378. doi: 10.1111/j.1699-0463.1972.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E. Atypical germ cells in the adjacent "normal" tissue of testicular tumours. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):127–130. doi: 10.1111/j.1699-0463.1975.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E. Possible carcinoma-in-situ of the testis. Lancet. 1972 Sep 9;2(7776):516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973 Jan;50(1):235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. Environmental influence on experimental teratocarcinogenesis in testes of mice. J Exp Zool. 1970 Aug;174(4):407–414. doi: 10.1002/jez.1401740405. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967 Apr;38(4):549–552. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Macaskill I. A., Currie A. R. Adrenocortical cell deletion: the role of ACTH. J Pathol. 1973 Oct;111(2):85–94. doi: 10.1002/path.1711110203. [DOI] [PubMed] [Google Scholar]
- Zamboni L., Merchant H. The fine morphology of mouse primordial germ cells in extragonadal locations. Am J Anat. 1973 Jul;137(3):299–335. doi: 10.1002/aja.1001370305. [DOI] [PubMed] [Google Scholar]