Abstract
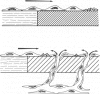
Endothelial coverage of an exposed synthetic vascular graft surface limits thrombosis and may improve long-term graft performance. In most types of synthetic graft, luminal endothelium is derived from the cut edges of adjacent artery. In this study the authors investigated the possibility that endothelial coverage could also be obtained by ingrowth of capillaries from the outside of the graft. Porous 4-mm polytetrafluorethylene (PTFE; 60 mu internodal distance) grafts were inserted into the aortoiliac circulation of baboons and were retrieved at intervals of up to 12 weeks. Between 1 and 2 weeks after surgery a continuous sheet of cells began to appear on the surface along the entire graft. These cells stained for Factor VIII related antigen, exhibited endothelial morphology by scanning electron microscopy, were associated with capillary orifices at the luminal surface, and covered the entire graft by 4 weeks. Transmural capillaries were observed to connect the graft lumen to extravascular granulation tissue. Despite full coverage of the graft, endothelial cells continued to exhibit increased proliferation (thymidine labeling) at 12 weeks. Smooth muscle cells (pericytes) accompanied capillary endothelium into the graft lumen, exhibited vascular smooth-muscle-specific immunostaining, and proliferated under the luminal endothelium to form intima. These results indicate that under some circumstances capillary endothelium and smooth muscle cells can function in the same manner as large vessel endothelium and smooth muscle and can provide rapid coverage of porous synthetic graft surfaces in contact with the arterial circulation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. T., Long J. A., Clark R. E., Sicard G. A., Hopkins K. T., Welch M. J. Influence of endothelial cell seeding on platelet deposition and patency in small-diameter Dacron arterial grafts. J Vasc Surg. 1984 Jan;1(1):224–233. [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel W. E., Vinter D. W., Ford J. W., Kahn R. H., Graham L. M., Stanley J. C. Sequential studies of healing in endothelial seeded vascular prostheses: histologic and ultrastructure characteristics of graft incorporation. J Surg Res. 1981 Apr;30(4):305–324. doi: 10.1016/0022-4804(81)90165-7. [DOI] [PubMed] [Google Scholar]
- Clagett G. P., Burkel W. E., Sharefkin J. B., Ford J. W., Hufnagel H., Vinter D. W., Kahn R. H., Graham L. M., Stanley J. C., Ramwell P. W. Platelet reactivity in vivo in dogs with arterial prostheses seeded with endothelial cells. Circulation. 1984 Mar;69(3):632–639. doi: 10.1161/01.cir.69.3.632. [DOI] [PubMed] [Google Scholar]
- Clagett G. P., Graeber G. M., Robinowitz M., Langloss J. M., Ramwell P. W. Differentiation of vascular prostheses in dogs with serial tests of in vivo platelet reactivity. Surgery. 1984 Mar;95(3):331–338. [PubMed] [Google Scholar]
- Clowes A. W., Gown A. M., Hanson S. R., Reidy M. A. Mechanisms of arterial graft failure. 1. Role of cellular proliferation in early healing of PTFE prostheses. Am J Pathol. 1985 Jan;118(1):43–54. [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Delvos U., Gajdusek C., Sage H., Harker L. A., Schwartz S. M. Interactions of vascular wall cells with collagen gels. Lab Invest. 1982 Jan;46(1):61–72. [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- FLOREY H. W., GREER S. J., KISER J., POOLE J. C., TELANDER R., WERTHESSEN N. T. The development of the pseudointima lining fabric grafts of the aorta. Br J Exp Pathol. 1962 Dec;43:655–660. [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Regulation of production of a platelet-derived growth factor-like protein by cultured bovine aortic endothelial cells. J Cell Physiol. 1984 Nov;121(2):298–308. doi: 10.1002/jcp.1041210206. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni J. J., Liotta D., Hall C. W., Adams J. G., Lechter A., Barrionueva M., O'Neal R. M., Debakey M. E. Healing of Pseudointimas in Velour-lined, Impermeable Arterial Prostheses. Am J Pathol. 1968 Sep;53(3):375–389. [PMC free article] [PubMed] [Google Scholar]
- Goldman M., Norcott H. C., Hawker R. J., Drolc Z., McCollum C. N. Platelet accumulation on mature Dacron grafts in man. Br J Surg. 1982 Jun;69 (Suppl):S38–S40. doi: 10.1002/bjs.1800691314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Sauvage L. R. Platelet consumption by arterial prostheses: the effects of endothelialization and pharmacologic inhibition of platelet function. Ann Surg. 1977 Nov;186(5):594–601. doi: 10.1097/00000658-197711000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring M. B., Dilley R., Jersild R. A., Jr, Boxer L., Gardner A., Glover J. Seeding arterial prostheses with vascular endothelium. The nature of the lining. Ann Surg. 1979 Jul;190(1):84–90. doi: 10.1097/00000658-197907000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring M., Baughman S., Glover J., Kesler K., Jesseph J., Campbell J., Dilley R., Evan A., Gardner A. Endothelial seeding of Dacron and polytetrafluoroethylene grafts: the cellular events of healing. Surgery. 1984 Oct;96(4):745–755. [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Synthesis of factor VIII by endothelial cells. Ann N Y Acad Sci. 1982;401:163–170. doi: 10.1111/j.1749-6632.1982.tb25715.x. [DOI] [PubMed] [Google Scholar]
- Jaye M., McConathy E., Drohan W., Tong B., Deuel T., Maciag T. Modulation of the sis gene transcript during endothelial cell differentiation in vitro. Science. 1985 May 17;228(4701):882–885. doi: 10.1126/science.3890179. [DOI] [PubMed] [Google Scholar]
- Kadish J. L., Butterfield C. E., Folkman J. The effect of fibrin on cultured vascular endothelial cells. Tissue Cell. 1979;11(1):99–108. doi: 10.1016/0040-8166(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Kusaba A., Fischer C. R., 3rd, Matulewski T. J., Matsumoto T. Experimental study of the influence of porosity on development of neointima in Gore-Tex grafts: a method to increase long-term patency rate. Am Surg. 1981 Aug;47(8):347–354. [PubMed] [Google Scholar]
- MACKENZIE D. C., LOEWENTHAL J. Endothelial growth in nylon vascular grafts. Br J Surg. 1960 Sep;48:212–217. doi: 10.1002/bjs.18004820827. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Hannan G. N., Reilly W. Signals causing change in morphological phenotype, growth mode, and gene expression of vascular endothelial cells. J Cell Physiol. 1982 Jul;112(1):96–106. doi: 10.1002/jcp.1041120115. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Hannan G. N., Reilly W., Stewart F. H. Variant endothelial cells. Fibronectin as a transducer of signals for migration and neovascularisation. J Cell Physiol. 1980 Aug;104(2):177–186. doi: 10.1002/jcp.1041040207. [DOI] [PubMed] [Google Scholar]
- McCollum C. N., Kester R. C., Rajah S. M., Learoyd P., Pepper M. Arterial graft maturation: the duration of thrombotic activity in Dacron aortobifemoral grafts measured by platelet and fibrinogen kinetics. Br J Surg. 1981 Jan;68(1):61–64. [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: a question of endothelial integrity and growth control of smooth muscle. Harvey Lect. 1981 1982;77:161–182. [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Selden S. C., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Haudenschild C. C., Eddy E. M. Endothelial regneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest. 1978 May;38(5):568–580. [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Stanley J. C., Burkel W. E., Ford J. W., Vinter D. W., Kahn R. H., Whitehouse W. M., Jr, Graham L. M. Enhanced patency of small-diameter, externally supported Dacron iliofemoral grafts seeded with endothelial cells. Surgery. 1982 Dec;92(6):994–1005. [PubMed] [Google Scholar]
- Stratton J. R., Thiele B. L., Ritchie J. L. Platelet deposition on Dacron aortic bifurcation grafts in man: quantitation with indium-111 platelet imaging. Circulation. 1982 Dec;66(6):1287–1293. doi: 10.1161/01.cir.66.6.1287. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Wesolowski S. A. Performance of materials as prosthetic blood vessels. Bull N Y Acad Med. 1972 Feb;48(2):331–356. [PMC free article] [PubMed] [Google Scholar]
- YONG N. K., KINMONTH J. B., TAYLOR G. W. THE ENDOTHELIAL LINING OF VASCULAR GRAFTS. Surg Gynecol Obstet. 1963 Sep;117:305–310. [PubMed] [Google Scholar]