Abstract
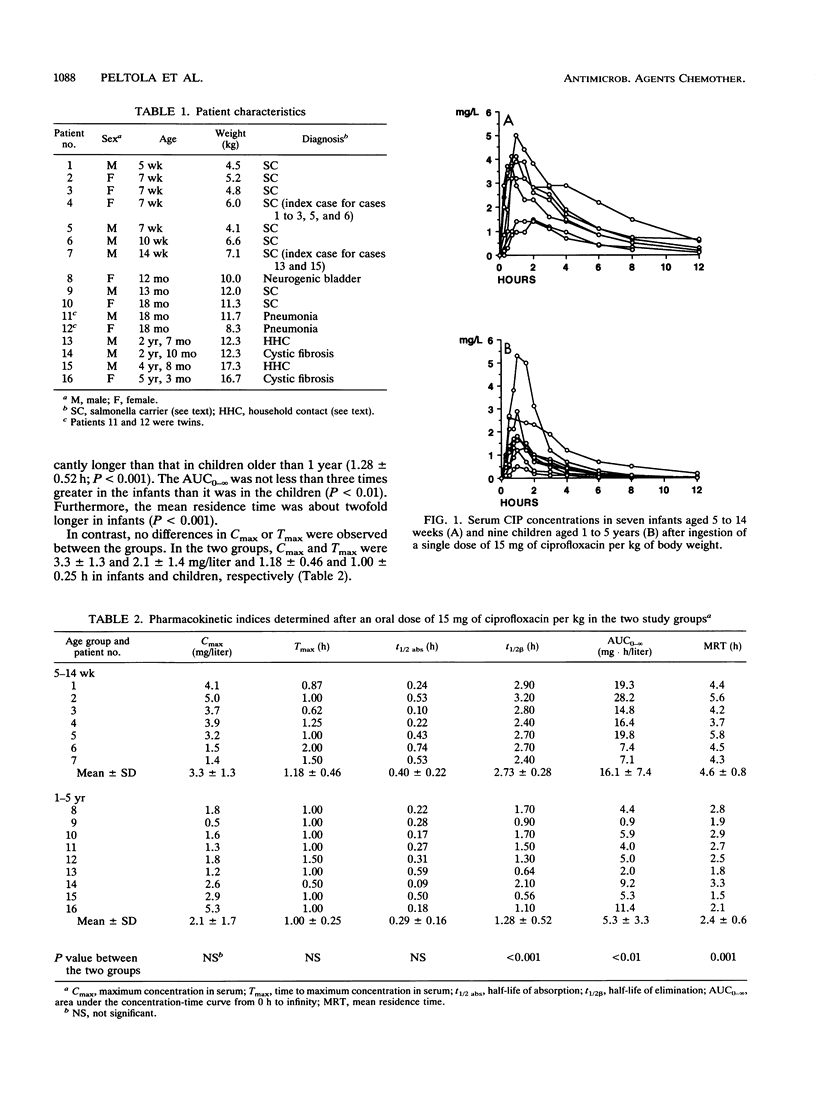
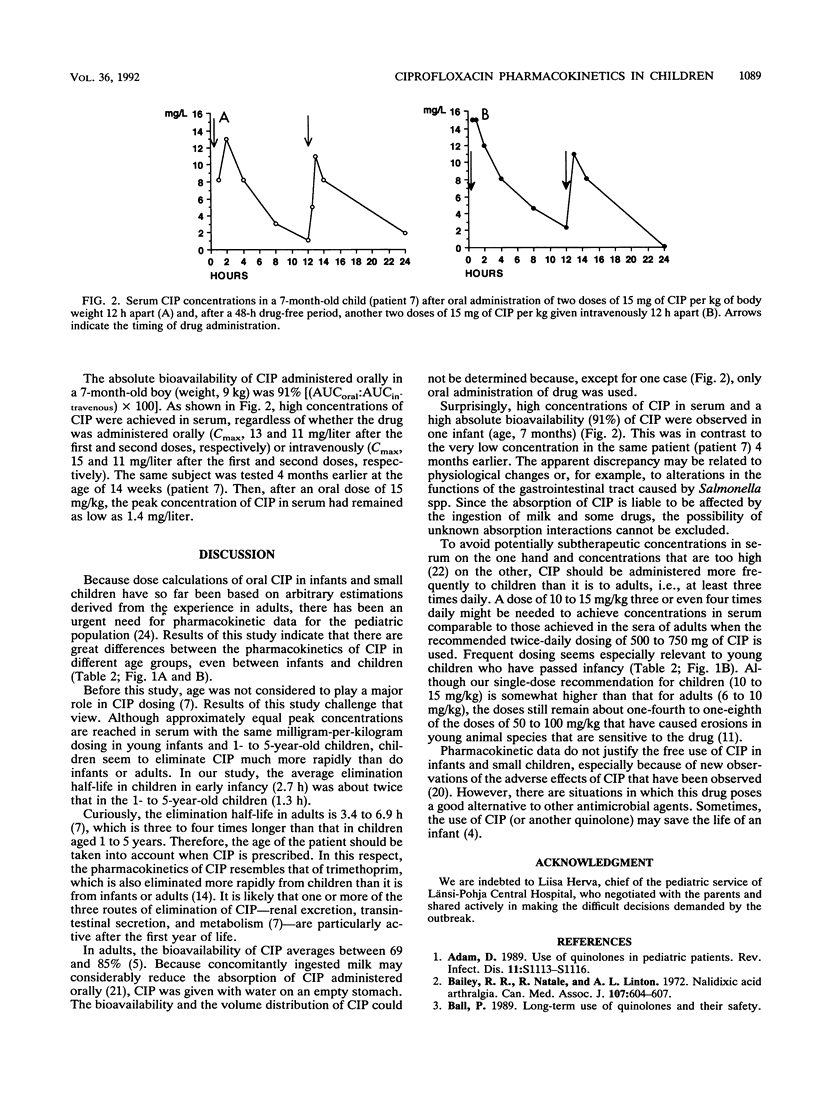
The pharmacokinetics of orally administered ciprofloxacin (CIP) was studied in seven infants aged 5 to 14 weeks and nine children aged 1 to 5 years, most of whom were Salmonella carriers. In each case, 15 mg of CIP per kg of body weight was given with water on an empty stomach, and timed serum samples were taken during the following 12 h. The elimination half-life of CIP was significantly (P less than 0.001) longer in the infants (2.73 +/- 0.28 h; mean +/- standard deviation) than it was in the children (1.28 +/- 0.52 h). The area under the serum CIP concentration-time curve (AUC) from time zero to infinity was 16.1 +/- 7.4 mg.h.liter-1 among the infants and 5.3 +/- 3.3 mg.h.liter-1 in the children (P less than 0.01). No significant differences in the maximum concentration in serum, time to maximum concentration in serum, or absorption half-life were observed between the two groups. In contrast, the mean residence time was twofold longer in the infants (4.6 h) than it was in the children (2.4 h; P less than 0.001). The findings suggest that elimination of CIP is particularly rapid in children who just have passed infancy; they may require doses at shorter time intervals than those required by infants or older children or adults. In general, an oral dose of 10 to 15 mg of CIP per kg three times daily seems appropriate for children aged 1 to 5 years.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam D. Use of quinolones in pediatric patients. Rev Infect Dis. 1989 Jul-Aug;11 (Suppl 5):S1113–S1116. doi: 10.1093/clinids/11.supplement_5.s1113. [DOI] [PubMed] [Google Scholar]
- Bailey R. R., Natale R., Linton A. L. Nalidixic acid arthralgia. Can Med Assoc J. 1972 Oct 7;107(7):604–passim. [PMC free article] [PubMed] [Google Scholar]
- Bannon M. J., Stutchfield P. R., Weindling A. M., Damjanovic V. Ciprofloxacin in neonatal Enterobacter cloacae septicaemia. Arch Dis Child. 1989 Oct;64(10 Spec No):1388–1391. doi: 10.1136/adc.64.10_spec_no.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Dalhoff A., Rohwedder R. Pharmacokinetics of ciprofloxacin. Infection. 1988;16 (Suppl 1):S3–13. doi: 10.1007/BF01650500. [DOI] [PubMed] [Google Scholar]
- Bouissou H., Caujolle D., Caujolle F., Milhaud G. Tissus cartilagineux et acide nalidixique. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jun 12;286(23):1743–1746. [PubMed] [Google Scholar]
- Campoli-Richards D. M., Monk J. P., Price A., Benfield P., Todd P. A., Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988 Apr;35(4):373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- Cheesbrough J. S., Mwema F. I., Green S. D., Tillotson G. S. Quinolones in children with invasive salmonellosis. Lancet. 1991 Jul 13;338(8759):127–127. doi: 10.1016/0140-6736(91)90127-b. [DOI] [PubMed] [Google Scholar]
- Chevais M., Reinert P., Rondeau M. C., Tobelem R., Albengres E., Riant P., Tillement J. P. Critical risk/benefit analysis of pefloxacine use in children under 15 years--the problem of arthralgias. Int J Clin Pharmacol Ther Toxicol. 1987 Jun;25(6):306–309. [PubMed] [Google Scholar]
- Douidar S. M., Snodgrass W. R. Potential role of fluoroquinolones in pediatric infections. Rev Infect Dis. 1989 Nov-Dec;11(6):878–889. doi: 10.1093/clinids/11.6.878. [DOI] [PubMed] [Google Scholar]
- Gough A., Barsoum N. J., Mitchell L., McGuire E. J., de la Iglesia F. A. Juvenile canine drug-induced arthropathy: clinicopathological studies on articular lesions caused by oxolinic and pipemidic acids. Toxicol Appl Pharmacol. 1979 Oct;51(1):177–187. doi: 10.1016/0041-008x(79)90020-6. [DOI] [PubMed] [Google Scholar]
- Hoppu K. Changes in trimethoprim pharmacokinetics after the newborn period. Arch Dis Child. 1989 Mar;64(3):343–345. doi: 10.1136/adc.64.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwen R. H., Bijleveld C. M., de Vries-Hospers H. G. Ciprofloxacin for cholangitis after hepatic portoenterostomy. Lancet. 1987 Jun 13;1(8546):1367–1367. doi: 10.1016/s0140-6736(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Isaacs D., Slack M. P., Wilkinson A. R., Westwood A. W. Successful treatment of Pseudomonas ventriculitis with ciprofloxacin. J Antimicrob Chemother. 1986 Apr;17(4):535–538. doi: 10.1093/jac/17.4.535. [DOI] [PubMed] [Google Scholar]
- Kiess W., Haas R., Marget W. Chloramphenicol-resistant Salmonella tennessee osteomyelitis. Infection. 1984 Sep-Oct;12(5):359–359. doi: 10.1007/BF01651156. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P., Pengsaa K., Sookpranee T. Ciprofloxacin in neonates and its possible adverse effect on the teeth. Pediatr Infect Dis J. 1991 Aug;10(8):619–620. doi: 10.1097/00006454-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Neuvonen P. J., Kivistö K. T., Lehto P. Interference of dairy products with the absorption of ciprofloxacin. Clin Pharmacol Ther. 1991 Nov;50(5 Pt 1):498–502. doi: 10.1038/clpt.1991.174. [DOI] [PubMed] [Google Scholar]
- Rahm V., Schacht P. Safety of ciprofloxacin. A review. Scand J Infect Dis Suppl. 1989;60:120–128. [PubMed] [Google Scholar]
- Schaad U. B., Stoupis C., Wedgwood J., Tschaeppeler H., Vock P. Clinical, radiologic and magnetic resonance monitoring for skeletal toxicity in pediatric patients with cystic fibrosis receiving a three-month course of ciprofloxacin. Pediatr Infect Dis J. 1991 Oct;10(10):723–729. [PubMed] [Google Scholar]
- Schaad U. B. Use of quinolones in pediatrics. Eur J Clin Microbiol Infect Dis. 1991 Apr;10(4):355–360. doi: 10.1007/BF01967011. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J., Guenin K., Buehlmann U., Kraemer R. Antipseudomonal therapy in cystic fibrosis: aztreonam and amikacin versus ceftazidime and amikacin administered intravenously followed by oral ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):858–865. doi: 10.1007/BF01963771. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J. Nalidixic acid in children: retrospective matched controlled study for cartilage toxicity. Infection. 1987 May-Jun;15(3):165–168. doi: 10.1007/BF01646040. [DOI] [PubMed] [Google Scholar]


