Abstract
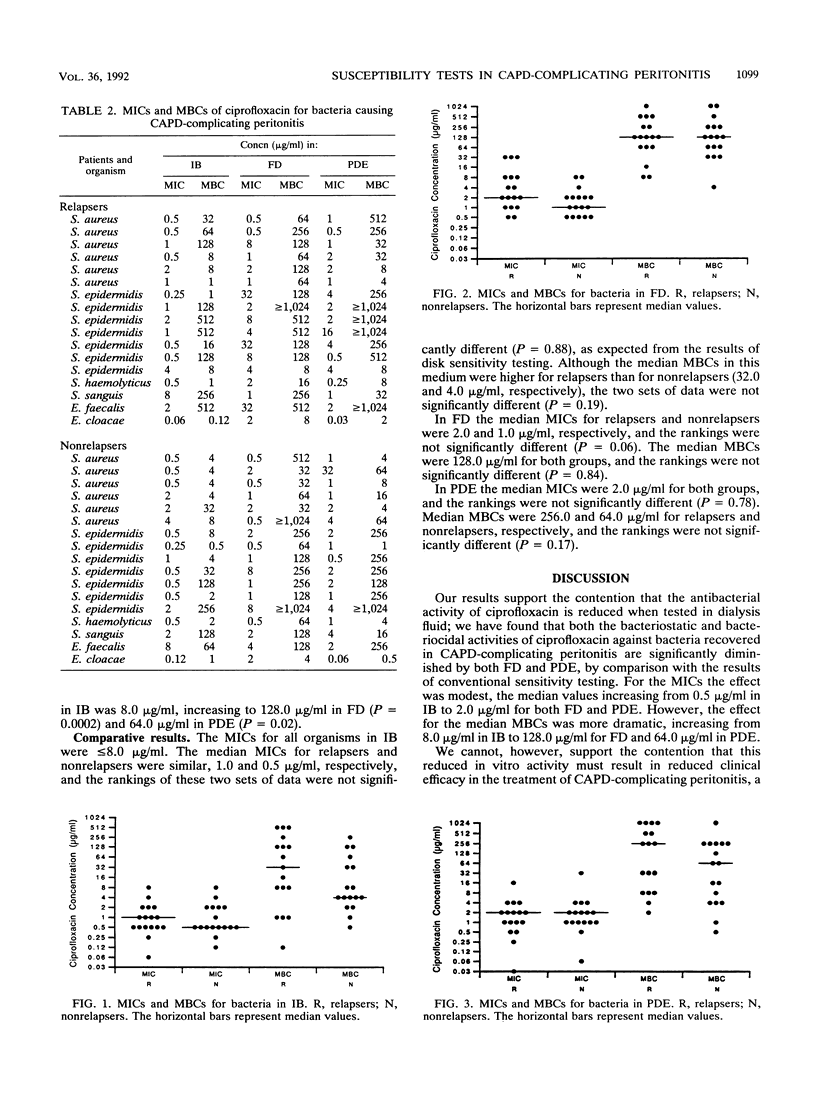
Antagonism of antibiotic activity by peritoneal dialysate has been postulated to be a cause of failure of treatment of peritonitis complicating continuous ambulatory peritoneal dialysis. We evaluated by a case-control study whether unexpected treatment failure could be attributed to such antagonism. Bacteria isolated from 34 patient episodes of peritonitis treated with the same regimen of ciprofloxacin monotherapy were studied. Ciprofloxacin was significantly less active in dialysate than in Iso-Sensitest broth (IB). The median MIC in IB was 0.5 microgram/ml, increasing to 2.0 micrograms/ml for both fresh dialysate (FD) (P = 0.003) and pooled dialysis effluent (PDE) (P = 0.03); the median MBC in IB was 8.0 micrograms/ml, increasing to 128.0 micrograms/ml in FD (P = 0.0002) and 64.0 micrograms/ml in PDE (P = 0.02). However, no significant differences were found in the results for patients suffering unexpected treatment failure (relapse of peritonitis) compared with the results for patients whose infection resolved without sequel. In IB the median MICs for relapsers and nonrelapsers were 1.0 and 0.5 microgram/ml, respectively (P = 0.88); median MBCs were 32.0 and 4.0 micrograms/ml (P = 0.19). In FD median MICs for relapsers and nonrelapsers were 2.0 and 1.0 micrograms/ml (P = 0.06); median MBCs were 128.0 micrograms/ml for both groups (P = 0.84). In PDE the median MICs were 2.0 micrograms/ml for both groups (P = 0.78); median MBCs were 256.0 and 64.0 micrograms/ml (P = 0.17). We therefore found no evidence to suggest that antagonism of antibiotic activity by dialysate is a cause of treatment failure or that conventional methods for laboratory susceptibility testing in peritonitis complicating continuous ambulatory peritoneal dialysis should be abandoned in favor of testing in media containing dialysate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Dasgupta M. K., Costerton J. W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990 Nov;34(11):2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby D. H., John J. F., Jr Effect of peritoneal dialysis solution on the antimicrobial activity of cephalosporins. Nephron. 1982;30(4):341–344. doi: 10.1159/000182513. [DOI] [PubMed] [Google Scholar]
- Buggy B. P., Schaberg D. R., Swartz R. D. Intraleukocytic sequestration as a cause of persistent Staphylococcus aureus peritonitis in continuous ambulatory peritoneal dialysis. Am J Med. 1984 Jun;76(6):1035–1040. doi: 10.1016/0002-9343(84)90854-4. [DOI] [PubMed] [Google Scholar]
- CAPD peritonitis. Lancet. 1987 Jun 6;1(8545):1320–1321. [PubMed] [Google Scholar]
- Craddock C. F., Edwards R., Finch R. G. Pseudomonas peritonitis in continuous ambulatory peritoneal dialysis: laboratory predictors of treatment failure. J Hosp Infect. 1987 Sep;10(2):179–186. doi: 10.1016/0195-6701(87)90145-9. [DOI] [PubMed] [Google Scholar]
- Diagnosis and management of peritonitis in continuous ambulatory peritoneal dialysis. Report of a working party of the British Society for Antimicrobial Chemotherapy. Lancet. 1987 Apr 11;1(8537):845–849. [PubMed] [Google Scholar]
- Edwards R., Thompson R. C., Finch R. G. Antibiotic susceptibility of staphylococci from CAPD peritonitis in children. J Antimicrob Chemother. 1987 Jul;20(1):138–139. doi: 10.1093/jac/20.1.138. [DOI] [PubMed] [Google Scholar]
- Evans R. C., Holmes C. J. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother. 1987 Jun;31(6):889–894. doi: 10.1128/aac.31.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eykyn S. J. Staphylococcal sepsis. The changing pattern of disease and therapy. Lancet. 1988 Jan 16;1(8577):100–104. doi: 10.1016/s0140-6736(88)90294-2. [DOI] [PubMed] [Google Scholar]
- Gray H. H., Goulding S., Eykyn S. J. Intraperitoneal vancomycin and ceftazidime in the treatment of CAPD peritonitis. Clin Nephrol. 1985 Feb;23(2):81–84. [PubMed] [Google Scholar]
- Greenwood D. Unrealistic nature of the 'MIC'. J Antimicrob Chemother. 1976 Sep;2(3):312–313. doi: 10.1093/jac/2.3.312. [DOI] [PubMed] [Google Scholar]
- Grise G., Lemeland J. F., Fillastre J. P. Etude de la stabilité de huit céphalosporines de deuxième et troisième générations dans le liquide de dialyse péritonéale. Pathol Biol (Paris) 1985 May;33(5):335–339. [PubMed] [Google Scholar]
- Guay D., Klicker R., Pence T., Peterson P. In vitro antistaphylococcal activity of teicoplanin and ciprofloxacin in peritoneal dialysis effluent. Eur J Clin Microbiol. 1986 Dec;5(6):661–663. doi: 10.1007/BF02013294. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Towards greater uniformity in sensitivity testing. J Antimicrob Chemother. 1977 Sep;3(5):385–392. doi: 10.1093/jac/3.5.385. [DOI] [PubMed] [Google Scholar]
- Lamperi S., Carozzi S. Immunological defenses in CAPD. Blood Purif. 1989;7(2-3):126–143. doi: 10.1159/000169585. [DOI] [PubMed] [Google Scholar]
- Ludlam H. A., Barton I., White L., McMullin C., King A., Phillips I. Intraperitoneal ciprofloxacin for the treatment of peritonitis in patients receiving continuous ambulatory peritoneal dialysis (CAPD). J Antimicrob Chemother. 1990 May;25(5):843–851. doi: 10.1093/jac/25.5.843. [DOI] [PubMed] [Google Scholar]
- Ludlam H. A. Infectious consequences of continuous ambulatory peritoneal dialysis. J Hosp Infect. 1991 Jun;18 (Suppl A):341–354. doi: 10.1016/0195-6701(91)90041-6. [DOI] [PubMed] [Google Scholar]
- Ludlam H. A., Noble W. C., Marples R. R., Phillips I. The evaluation of a typing scheme for coagulase-negative staphylococci suitable for epidemiological studies. J Med Microbiol. 1989 Nov;30(3):161–165. doi: 10.1099/00222615-30-3-161. [DOI] [PubMed] [Google Scholar]
- Ludlam H. A., Price T. N., Berry A. J., Phillips I. Laboratory diagnosis of peritonitis in patients on continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1988 Sep;26(9):1757–1762. doi: 10.1128/jcm.26.9.1757-1762.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam H. A., Young A. E., Berry A. J., Phillips I. The prevention of infection with Staphylococcus aureus in continuous ambulatory peritoneal dialysis. J Hosp Infect. 1989 Nov;14(4):293–301. doi: 10.1016/0195-6701(89)90069-8. [DOI] [PubMed] [Google Scholar]
- McCormick E. M., Echols R. M. Effect of peritoneal dialysis fluid and pH on bactericidal activity of ciprofloxacin. Antimicrob Agents Chemother. 1987 Apr;31(4):657–659. doi: 10.1128/aac.31.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin C., Bouchet J. J., Gaillard I., Bebear C. Stability of fosfomycin and quinolones in peritoneal dialysis solution. J Antimicrob Chemother. 1990 May;25(5):878–880. doi: 10.1093/jac/25.5.878. [DOI] [PubMed] [Google Scholar]
- Selwyn S. Unrealistic nature of the "MIC". J Antimicrob Chemother. 1976 Jun;2(2):221–222. doi: 10.1093/jac/2.2.221. [DOI] [PubMed] [Google Scholar]
- Shalit I., Welch D. F., San Joaquin V. H., Marks M. I. In vitro antibacterial activities of antibiotics against Pseudomonas aeruginosa in peritoneal dialysis fluid. Antimicrob Agents Chemother. 1985 Jun;27(6):908–911. doi: 10.1128/aac.27.6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. C. Infections in continuous ambulatory peritoneal dialysis. J Med Microbiol. 1988 Sep;27(1):1–9. doi: 10.1099/00222615-27-1-1. [DOI] [PubMed] [Google Scholar]
- Venditti M., Santini C., Serra P., Micozzi A., Gentile G., Martino P. Comparative in vitro activities of new fluorinated quinolones and other antibiotics against coagulase-negative Staphylococcus blood isolates from neutropenic patients, and relationship between susceptibility and slime production. Antimicrob Agents Chemother. 1989 Feb;33(2):209–211. doi: 10.1128/aac.33.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Conroy W. E., Peterson P. K. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1984 Aug;20(2):199–203. doi: 10.1128/jcm.20.2.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissauer-Condon C., Engels I., Daschner F. D. In vitro activity of four new quinolones in Mueller-Hinton broth and peritoneal dialysis fluid. Eur J Clin Microbiol. 1987 Jun;6(3):324–326. doi: 10.1007/BF02017630. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Edwards R., Finch R. G. Laboratory studies on coagulase-negative staphylococci from CAPD-associated peritonitis. J Antimicrob Chemother. 1985 Mar;15(3):297–303. doi: 10.1093/jac/15.3.297. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Finch R. G. Ciprofloxacin and CAPD peritonitis. J Antimicrob Chemother. 1990 Sep;26(3):447–448. doi: 10.1093/jac/26.3.447. [DOI] [PubMed] [Google Scholar]


