Abstract
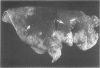
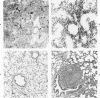
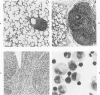
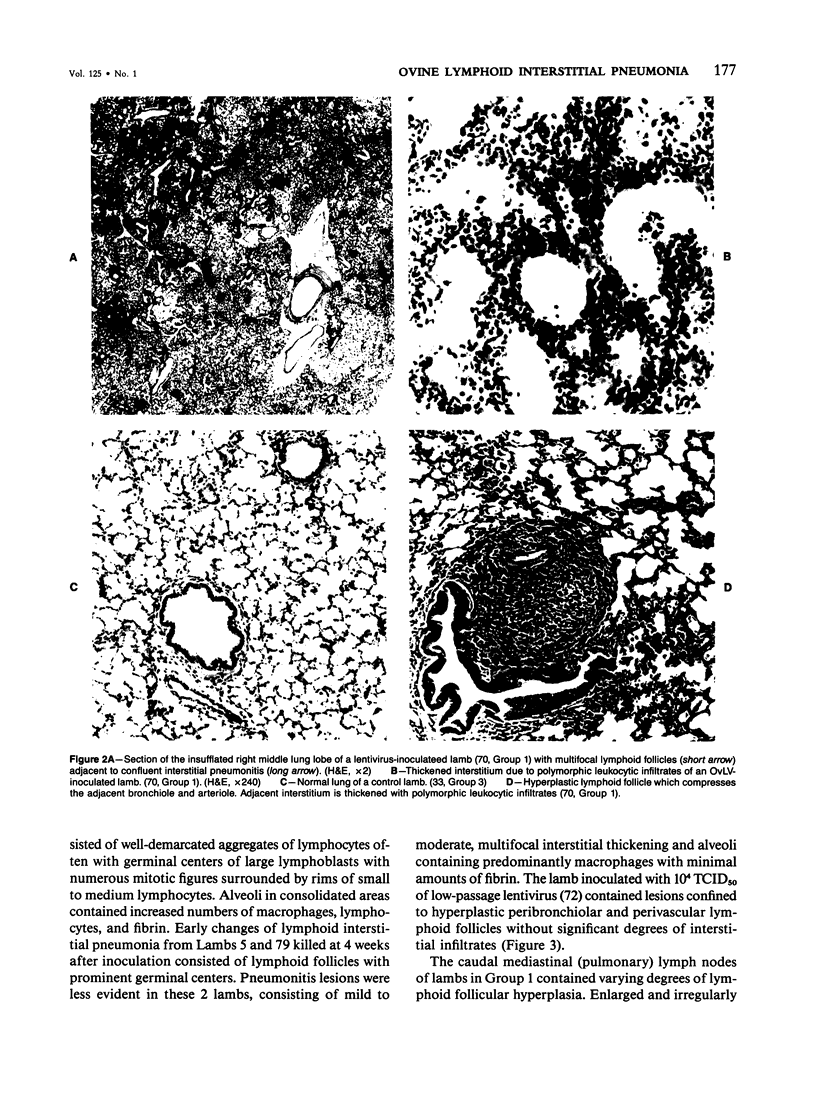
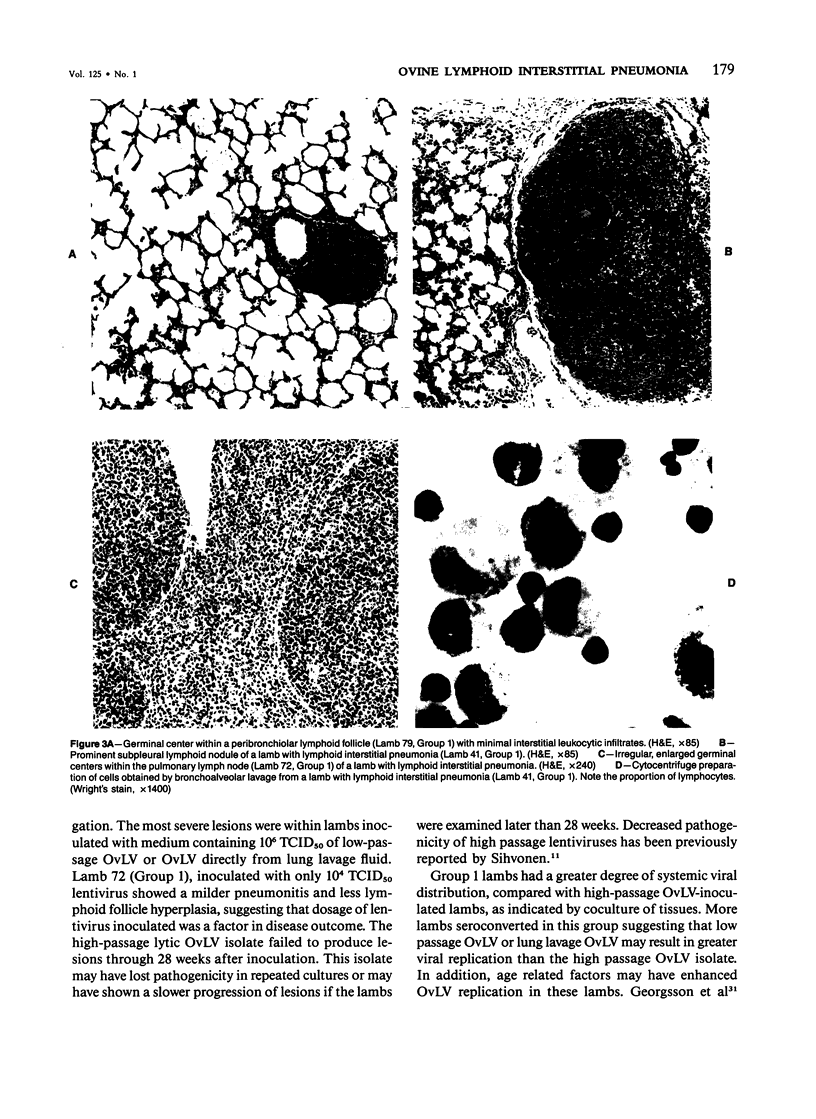
For examination of the characteristics of lentivirus-induced pulmonary disease in an animal model, neonatal lambs were given intratracheal injections of high-and low-passage ovine lentivirus (OvLV) isolates. In 6 of 6 lambs inoculated with low-passage OvLV or OvLV from lung lavage fluid, lesions of lymphoid interstitial pneumonia (LIP) developed. In none of 7 lambs inoculated with a high-passage OvLV or 4 control lambs inoculated with medium alone or ultrafiltered lung fluid did lung lesions develop. Systemic distribution of lentivirus was greater and development of lentivirus antibody was more rapid in lambs inoculated with low-passage OvLV, compared with lambs inoculated with high passage OvLV. The number of lymphocytes in bronchoalveolar lavage samples was increased in lambs with lymphoid interstitial pneumonia. The development of lymphoid interstitial pneumonia was markedly accelerated, in comparison with previous reports of experimentally induced lentivirus pneumonia in sheep. In lentivirus-inoculated lambs pulmonary lesions developed comparable to lymphoid interstitial pneumonia associated with the acquired immunodeficiency syndrome and other human benign lymphoid disorders of the lung. Similarities between the disease manifestations and virologic properties of OvLV and human T-cell lymphotropic virus III argue for the relevance of OvLV-induced disease as a model for human retrovirus diseases. The ability of OvLV to cause accelerated pulmonary disease in neonates may be due to age-related susceptibility factors that enhance the pathogenicity of lentiviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cutlip R. C., Jackson T. A., Laird G. A. Immunodiffusion test for ovine progressive pneumonia. Am J Vet Res. 1977 Jul;38(7):1081–1084. [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Laird G. A. Prevalence of ovine progressive pneumonia in a sampling of cull sheep from western and midwestern United States. Am J Vet Res. 1977 Dec;38(12):2091–2093. [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Lehmkuhl H. D. Lesions of ovine progressive pneumonia: interstitial pneumonitis and encephalitis. Am J Vet Res. 1979 Oct;40(10):1370–1374. [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Brogden K. A., McClurkin A. W. Vasculitis associated with ovine progressive pneumonia virus infection in sheep. Am J Vet Res. 1985 Jan;46(1):61–64. [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Evidence of decreased concanavalin A induced suppressor cell activity in the peripheral blood and pulmonary lymph nodes of sheep with ovine progressive pneumonia. Vet Immunol Immunopathol. 1985 Jan;8(1-2):93–106. doi: 10.1016/0165-2427(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Immunomorphologic and morphometric changes in pulmonary lymph nodes of sheep with progressive pneumonia. Vet Pathol. 1985 Jan;22(1):32–41. doi: 10.1177/030098588502200105. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Ovine interleukin-2: partial purification and assay in normal sheep and sheep with ovine progressive pneumonia. Vet Immunol Immunopathol. 1985 Jan;8(1-2):15–25. doi: 10.1016/0165-2427(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Fedullo A. J., Ettensohn D. B. Bronchoalveolar lavage in lymphangitic spread of adenocarcinoma to the lung. Chest. 1985 Jan;87(1):129–131. doi: 10.1378/chest.87.1.129. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Ventura P., Stowring L., Haase A. T. Quantitative analysis of visna virus replication in vivo. Virology. 1985 Feb;141(1):148–154. doi: 10.1016/0042-6822(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgsson G., Pétursson G., Miller A., Nathanson N., Pálsson P. A. Experimental visna in foetal Icelandic sheep. J Comp Pathol. 1978 Oct;88(4):597–605. doi: 10.1016/0021-9975(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Grieco M. H., Chinoy-Acharya P. Lymphocytic interstitial pneumonia associated with the acquired immune deficiency syndrome. Am Rev Respir Dis. 1985 Jun;131(6):952–955. doi: 10.1164/arrd.1985.131.6.952. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levinson W. Inhibition of RNA slow viruses by thiosemicarbazones. Biochem Biophys Res Commun. 1973 Apr 16;51(4):875–880. doi: 10.1016/0006-291x(73)90008-9. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Rojko J. L., Wilson P. L., Olsen R. G. Determinants of susceptibility and resistance to feline leukemia virus infection. I. Role of macrophages. J Natl Cancer Inst. 1981 Oct;67(4):889–898. doi: 10.1093/jnci/67.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell P. C., Luce J. M. Pulmonary involvement in the acquired immunodeficiency syndrome. Chest. 1985 Jan;87(1):104–112. doi: 10.1378/chest.87.1.104. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., König C. D., de Boer G. F., Schaake J., Jr Maedi-visna control in sheep. I. Artificial rearing of colostrum-deprived lambs. Vet Microbiol. 1983 Apr;8(2):179–185. doi: 10.1016/0378-1135(83)90064-0. [DOI] [PubMed] [Google Scholar]
- Huffman E. M., Kirk J. H., Winward L., Gorham J. R. Serologic prevalence of ovine progressive pneumonia in a western range flock of sheep. J Am Vet Med Assoc. 1981 Apr 1;178(7):708–710. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M., Minnefor A. B., Saad S., Klein K. M., Singh R., Zabala M., Dadzie C., Simpser M., Rapkin R. H. Pathologic pulmonary findings in children with the acquired immunodeficiency syndrome: a study of ten cases. Hum Pathol. 1985 Mar;16(3):241–246. doi: 10.1016/s0046-8177(85)80009-5. [DOI] [PubMed] [Google Scholar]
- Kradin R. L., Mark E. J. Benign lymphoid disorders of the lung, with a theory regarding their development. Hum Pathol. 1983 Oct;14(10):857–867. doi: 10.1016/s0046-8177(83)80161-0. [DOI] [PubMed] [Google Scholar]
- Larsen H. J., Hyllseth B., Krogsrud J. Experimental maedi virus infection in sheep: cellular and humoral immune response during three years following intranasal inoculation. Am J Vet Res. 1982 Mar;43(3):384–389. [PubMed] [Google Scholar]
- Marchevsky A., Rosen M. J., Chrystal G., Kleinerman J. Pulmonary complications of the acquired immunodeficiency syndrome: a clinicopathologic study of 70 cases. Hum Pathol. 1985 Jul;16(7):659–670. doi: 10.1016/s0046-8177(85)80148-9. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Light M. R., Schipper I. A. Elevated concentrations in serum immunoglobulins due to infection by ovine progressive pneumonia virus. Am J Vet Res. 1979 Jan;40(1):69–72. [PubMed] [Google Scholar]
- Oliver R. E., Gorham J. R., Parish S. F., Hadlow W. J., Narayan O. Ovine progressive pneumonia: pathologic and virologic studies on the naturally occurring disease. Am J Vet Res. 1981 Sep;42(9):1554–1559. [PubMed] [Google Scholar]
- Parks W. P., Scott G. B. Pediatric AIDS: a disease spectrum causally associated with HTLV-III infection. Cancer Res. 1985 Sep;45(9 Suppl):4659s–4661s. [PubMed] [Google Scholar]
- Pétursson G., Nathanson N., Georgsson G., Panitch H., Pálsson P. A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Invest. 1976 Oct;35(4):402–412. [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Filippi P., Vigne R., Russo P., Vitu C. Highly lytic and persistent lentiviruses naturally present in sheep with progressive pneumonia are genetically distinct. J Virol. 1984 Nov;52(2):672–679. doi: 10.1128/jvi.52.2.672-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A., TRYGGVADDOTTIR A. Transmission experiments with maedi. J Infect Dis. 1953 Sep-Oct;93(2):166–175. doi: 10.1093/infdis/93.2.166. [DOI] [PubMed] [Google Scholar]
- Saltini C., Crystal R. G. Pulmonary sarcoidosis: pathogenesis, staging and therapy. Int Arch Allergy Appl Immunol. 1985;76 (Suppl 1):92–100. doi: 10.1159/000233739. [DOI] [PubMed] [Google Scholar]
- Scott G. B., Buck B. E., Leterman J. G., Bloom F. L., Parks W. P. Acquired immunodeficiency syndrome in infants. N Engl J Med. 1984 Jan 12;310(2):76–81. doi: 10.1056/NEJM198401123100202. [DOI] [PubMed] [Google Scholar]
- Sharp J. M., Angus K. W., Gray E. W., Scott F. M. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch Virol. 1983;78(1-2):89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- Sihvonen L., Estola T., Tuomi J. Experimental maedi infection in sheep. 1. Detection of virus, clinical course, histopathology. Acta Vet Scand. 1980;21(1):113–123. doi: 10.1186/BF03546906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Ziza J. M., Brun-Vezinet F., Venet A., Rouzioux C. H., Traversat J., Israel-Biet B., Barre-Sinoussi F., Chermann J. C., Godeau P. Lymphadenopathy-associated virus isolated from bronchoalveolar lavage fluid in AIDS-related complex with lymphoid interstitial pneumonitis. N Engl J Med. 1985 Jul 18;313(3):183–183. doi: 10.1056/NEJM198507183130313. [DOI] [PubMed] [Google Scholar]