Abstract
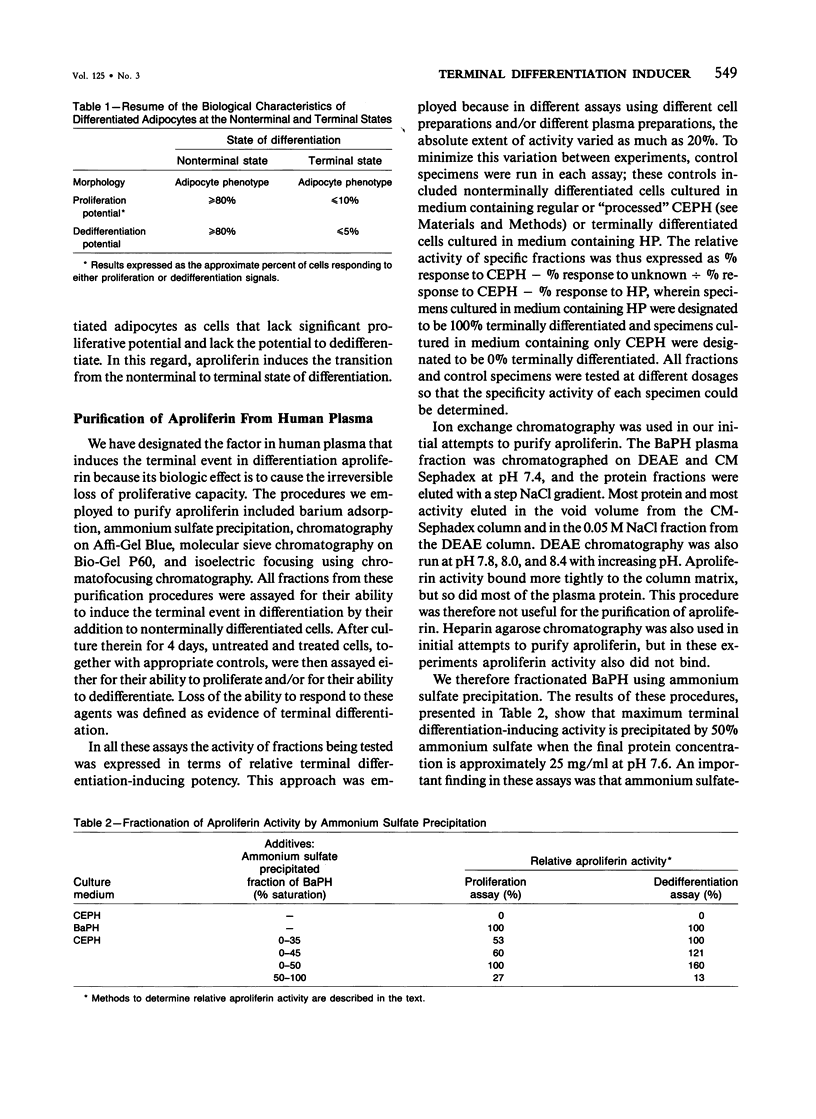
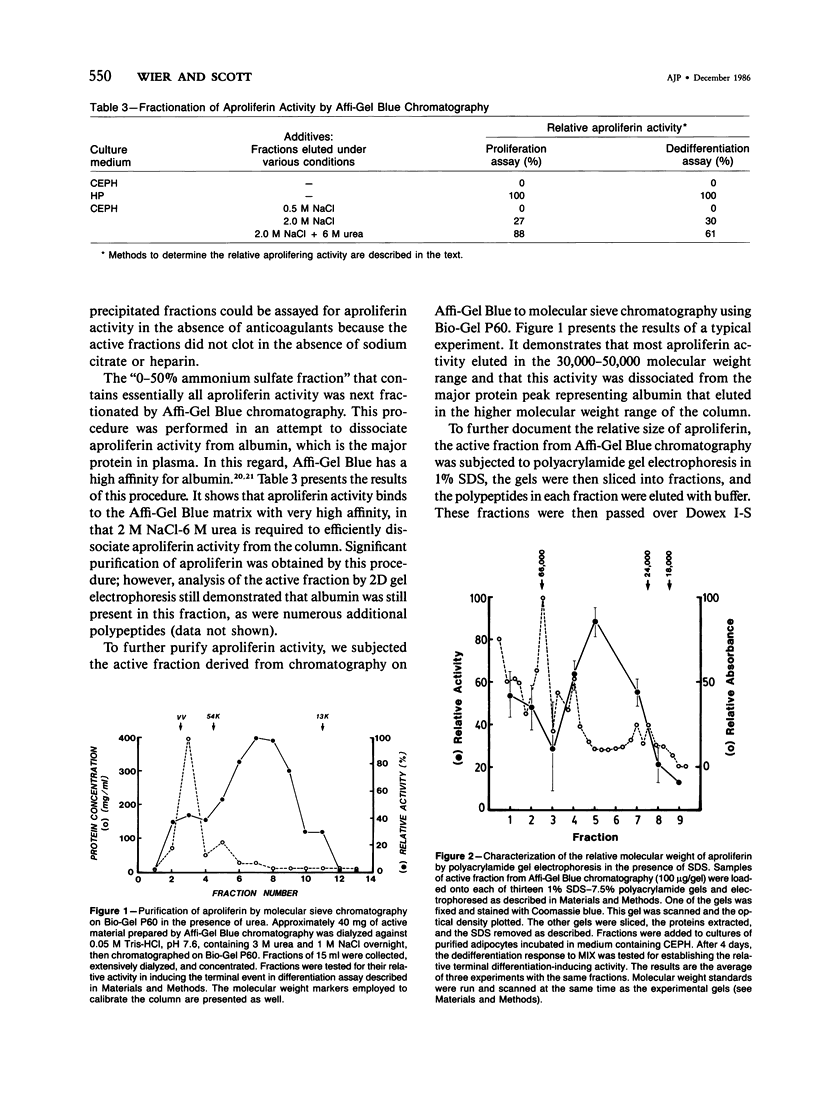
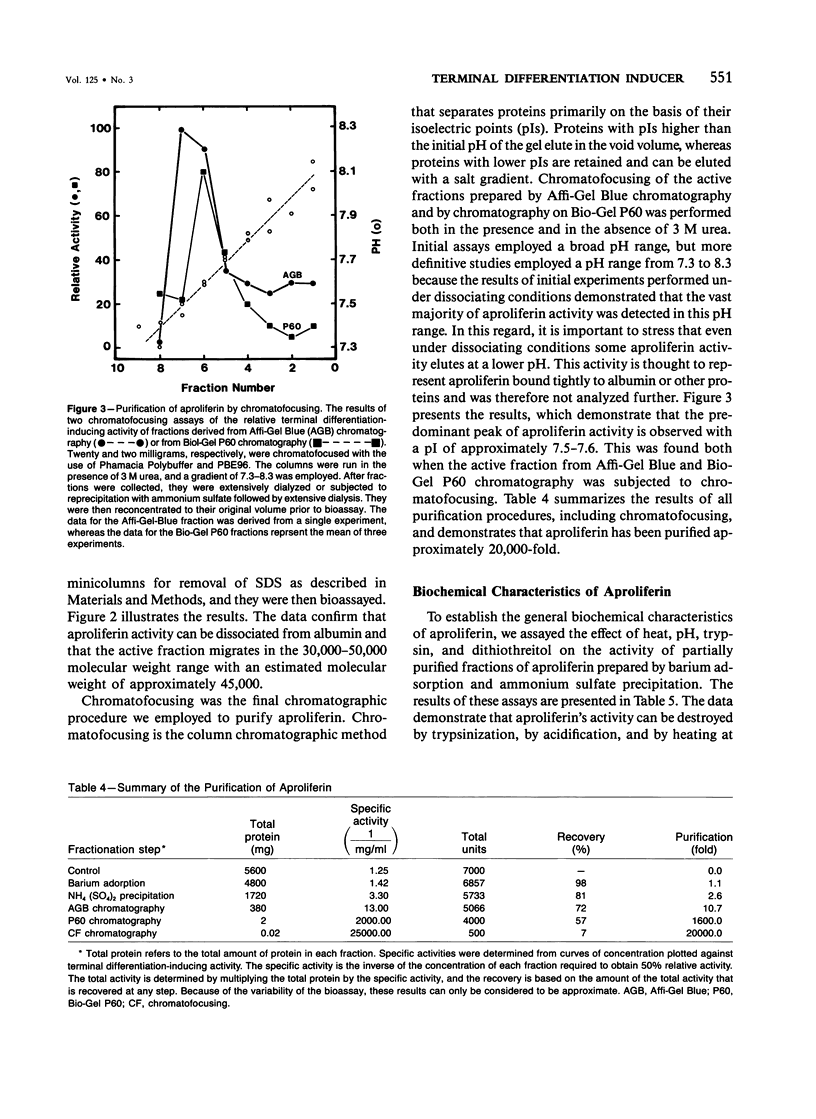
Cellular proliferation is regulated not only by the action of growth factors and growth inhibitors whose effects are reversible but also by factors that induce the irreversible loss of proliferative potential associated with the terminal event in cellular differentiation. The authors have employed 3T3 T mesenchymal stem cells as a model system to study the terminal event in cellular differentiation because in these cells' distinct nonterminal and terminal states of differentiation can be identified and because transition from the nonterminal to the terminal states of differentiation can be induced by human plasma. In this paper is reported the 20,000-fold purification of a component of human plasma that induces the terminal event in differentiation. This factor is shown to have an apparent molecular weight of approximately 45,000 and an isoelectric point of approximately 7.6. It is trypsin-sensitive, acid and heat-labile, and is resistant to treatment with dithiothreitol and alkali. The ability of this human plasma protein to induce the irreversible loss of proliferative potential associated with the terminal event in differentiation serves as the basis for its designation "aproliferin." The data in this paper in addition show that no other pharmacologic or physiologic agents have been identified that can mimic the biologic effect of aproliferin. Therefore, aproliferin appears to be a functionally distinct protein in human plasma.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett D. C. Differentiation in mouse melanoma cells: initial reversibility and an on-off stochastic model. Cell. 1983 Sep;34(2):445–453. doi: 10.1016/0092-8674(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Chen Z., Banks J., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc Natl Acad Sci U S A. 1982 Jan;79(2):471–475. doi: 10.1073/pnas.79.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A., Erard F., Schneider A. P., Schechter A. N. Induction of hemoglobin accumulation in human K562 cells by hemin is reversible. Science. 1981 Apr 24;212(4493):459–461. doi: 10.1126/science.6163216. [DOI] [PubMed] [Google Scholar]
- Devlin B. H., Konigsberg I. R. Reentry into the cell cycle of differentiated skeletal myocytes. Dev Biol. 1983 Jan;95(1):175–192. doi: 10.1016/0012-1606(83)90016-7. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- Gianazza E., Arnaud P. A general method for fractionation of plasma proteins. Dye-ligand affinity chromatography on immobilized Cibacron blue F3-GA. Biochem J. 1982 Jan 1;201(1):129–136. doi: 10.1042/bj2010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianazza E., Arnaud P. Chromatography of plasma proteins on immobilized Cibacron Blue F3-GA. Mechanism of the molecular interaction. Biochem J. 1982 Jun 1;203(3):637–641. doi: 10.1042/bj2030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz B. R., Scott R. E. Coupling of proadipocyte growth arrest and differentiation. I. Induction by heparinized medium containing human plasma. J Cell Biol. 1982 Aug;94(2):394–399. doi: 10.1083/jcb.94.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980 Nov;22(2 Pt 2):629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Hoerl B. J., Wille J. J., Jr, Florine D. L., Krawisz B. R., Yun K. Coupling of proadipocyte growth arrest and differentiation. II. A cell cycle model for the physiological control of cell proliferation. J Cell Biol. 1982 Aug;94(2):400–405. doi: 10.1083/jcb.94.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Maercklein P. B. An initiator of carcinogenesis selectively and stably inhibits stem cell differentiation: a concept that initiation of carcinogenesis involves multiple phases. Proc Natl Acad Sci U S A. 1985 May;82(9):2995–2999. doi: 10.1073/pnas.82.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Wille J. J., Jr, Pittelkow M. R., Sparks R. L. Biological mechanisms of stem cell carcinogenesis: a concept for multiple phases in the initiation of carcinogenesis and the role of differentiation control defects. Carcinog Compr Surv. 1985;9:67–80. [PubMed] [Google Scholar]
- Scott R. E., Wille J. J., Jr, Wier M. L. Mechanisms for the initiation and promotion of carcinogenesis: a review and a new concept. Mayo Clin Proc. 1984 Feb;59(2):107–117. doi: 10.1016/s0025-6196(12)60244-4. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Defective control of terminal differentiation and its role in carcinogenesis in the 3T3 T proadipocyte stem cell line. Cancer Res. 1985 Jul;45(7):3339–3346. [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Regulation of the terminal event in cellular differentiation: biological mechanisms of the loss of proliferative potential. J Cell Biol. 1986 May;102(5):1955–1964. doi: 10.1083/jcb.102.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen A., Fairchild D. G. T-cell control of B-cell proliferation uncoupled from differentiation. Cell Immunol. 1982 Dec;74(2):269–276. doi: 10.1016/0008-8749(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Morgan D. L. Mouse skin cells resistant to terminal differentiation associated with initiation of carcinogenesis. Nature. 1981 Sep 3;293(5827):72–74. doi: 10.1038/293072a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Schaer J. C., Muller D. E., Schneider J., Miodonski-Maculewicz N. M., Schindler R. Formation of mast cell granules in cell cycle mutants of an undifferentiated mastocytoma line: evidence for two different states of reversible proliferative quiescence. J Cell Biol. 1983 Jun;96(6):1756–1760. doi: 10.1083/jcb.96.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]


