Abstract
Pathways through which reticulospinal neurons can influence contralateral limb movements were investigated by recording from motoneurons innervating hindlimb muscles. Reticulospinal tract fibers were stimulated within the brainstem or in the lateral funiculus of the thoracic spinal cord contralateral to the motoneurons. Effects evoked by ipsilaterally descending reticulospinal tract fibers were eliminated by a spinal hemisection at an upper lumbar level. Stimuli applied in the brainstem evoked EPSPs, IPSPs, or both at latencies of 1.42 ± 0.03 and 1.53 ± 0.04 msec, respectively, from the first components of the descending volleys and with properties indicating a disynaptic linkage, in most contralateral motoneurons: EPSPs in 76% and IPSPs in 26%. EPSPs with characteristics of monosynaptically evoked responses, attributable to direct actions of crossed axon collaterals of reticulospinal fibers, were found in a small proportion of the motoneurons, whether evoked from the brainstem (9%) or from the thoracic cord (12.5%). Commissural neurons, which might mediate the crossed disynaptic actions (i.e., were antidromically activated from contralateral motor nuclei and monosynaptically excited from the ipsilateral reticular formation), were found in Rexed's lamina VIII in the midlumbar segments (L3–L5). The results reveal that although direct actions of reticulospinal fibers are much more potent on ipsilateral motoneurons, interneuronally mediated actions are as potent contralaterally as ipsilaterally, and midlumbar commissural neurons are likely to contribute to them. They indicate a close coupling between the spinal interneuronal systems used by the reticulospinal neurons to coordinate muscle contractions ipsilaterally and contralaterally.
Keywords: spinal cord, reticulospinal neurons, commissural neurons, motoneurons, interneurons, spinal neuronal networks
Introduction
Reticulospinal tract neurons have powerful actions in the spinal cord and are important for adjusting both posture and a variety of reflex and centrally initiated movements (Peterson et al., 1978; Peterson et al., 1979; Drew and Rossignol, 1990a,b; Grillner et al., 1995). They are considered to be particularly important for coordinated actions of the limbs and trunk. However, the spinal neuronal networks via which they act have been studied to a much smaller extent in mammals than in lower vertebrates. In the feline spinal cord, attention has been focused on ipsilateral neuronal networks (Lund and Pompeiano, 1965; Grillner et al., 1968;Lund and Pompeiano, 1968; Shapovalov, 1969; Wilson and Yoshida, 1969;Floeter et al., 1993). The actions of reticulospinal tract neurons on contralateral neurons have hardly been analyzed, even when reported (Floeter et al., 1993; Habaguchi et al., 2002).
In the present study, we have addressed three main questions related to crossed reticulospinal actions pertinent to their function in coordinating muscle activity on two sides of the body. The first was whether reticulospinal neurons induce monosynaptic EPSPs in contralateral lumbar motoneurons. The second was whether any disynaptic EPSPs or IPSPs are induced in these neurons as a counterpart of disynaptic reticulospinal actions on ipsilateral motoneurons (Lund and Pompeiano, 1965; Grillner et al., 1968; Lund and Pompeiano, 1968;Shapovalov, 1969; Wilson and Yoshida, 1969), and the third was to what extent any disynaptic PSPs evoked in contralateral motoneurons might be mediated by lamina VIII commissural neurons. Lamina VIII interneurons constitute the main group of neurons forming synaptic contacts with contralateral motoneurons (Scheibel and Scheibel, 1966; Harrison et al., 1986; Alstermark and Kummel, 1990; Hoover and Durkovic, 1992). Recently, some commissural neurons (including lamina VIII neurons) were found to be excited by stimuli applied in the pontomedullary reticular formation (RF), the mesencephalic and cerebellar locomotor regions from which reticulospinal neurons are activated (Jankowska and Noga, 1990;Matsuyama and Mori, 1998; Mori et al., 1998), or both. Neurons located in lamina VIII at some level in the lumbosacral enlargement have thus been considered very strong candidates for mediating any crossed disynaptic reticulospinal actions on motoneurons but are not the only candidates.
In view of the very complex projection patterns of reticulospinal neurons and interconnections between them (Mitani et al., 1988a,b,c) and the unavoidably widespread actions of stimuli applied within RF, the study could not be restricted to a particular population of RF neurons. However, the analysis has been restricted to synaptic actions mediated by reticulospinal tract fibers descending on only one side of the spinal cord. These were fibers on the same side as the stimulation sites, fibers on the side of location of motoneurons being transected at an upper lumbar level.
Materials and Methods
Preparation. The experiments were performed on 12 deeply anesthetized cats of both sexes (weighing 2.2–3.2 kg). Anesthesia was induced with sodium pentobarbital (40 mg/kg, i.p.) and maintained with intermittent doses of pentobarbital (1–2 mg/kg, i.v., up to a total dose of 45 mg/kg) and of α-chloralose (α-chloralose; Rhône Poulenc Santé; ∼5 mg · kg−1 · hr−1, i.v., up to a total dose of 55 mg/kg) at a level at which no withdrawal reflexes were present. During recording, neuromuscular transmission was blocked by pancuronium bromide (Pavulon; Organon Teknika, Askim, Sweden, ∼0.2 mg · kg−1 · hr−1, i.v.), and the animals were artificially respired. The depth of anesthesia was then assessed by continuously recording blood pressure and heart rate and by monitoring pupil diameter. If there was any increase in the blood pressure or heart rate, or if the pupils began to dilate, additional doses of chloralose were given. The mean blood pressure was kept at 100–130 mmHg, and the end-tidal concentration of CO2 was kept at ∼4% by adjusting parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 mg · kg−1 · hr−1). The core body temperature was kept at ∼38°C by servo-controlled infrared lamps. At the end of the experiment, the animals were killed with an overdose of anesthetic (until cardiac arrest) or by formalin perfusion. All the experimental procedures were approved by Göteborg ethics committee and followed National Institutes of Health and European Union guidelines of animal care.
A preliminary dissection included cannulation of the trachea (for artificial respiration and for a continuous monitoring of end-tidal CO2), a carotid artery (for continuous monitoring of blood pressure), and left and right cephalic veins (for intravenous injection of anesthetics and other fluids). A number of peripheral left and right hindlimb nerves were dissected, transected, and mounted on stimulating electrodes: either subcutaneous cuff electrodes [for the quadriceps (Q), sartorius (Sart), and gracilis nerves] or pairs of silver hook electrodes in a paraffin oil pool [for the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius and soleus (GS), plantaris, flexor digitorum and hallucis longus (FDL), the remaining part of the tibial (Tib) nerve, and tibialis anterior and extensor digitorum longus branches of the peroneal nerve, jointly referred to as the deep peroneal (DP)]. Laminectomies were performed at the level of the lower thoracic (T11–T13) and the upper lumbar to sacral (L2–S1) segments. The cerebellum was exposed to allow insertion of a stimulating electrode into the reticular formation. A hemisection of the spinal cord was performed at the level of the L2 segment, on the side of the motoneurons to be recorded from, and opposite to that of the reticular stimulation (as indicated in Fig. 1A,B). The dorsal columns were removed over a distance of a few millimeters; the central canal was visualized; and the tissue lateral to it was separated intrapially with watchmaker's forceps over a distance of 2–3 mm until the surface of the ventral funiculus on the opposite side was reached.
Fig. 1.
Possible substrates of oligosynaptic actions of reticulospinal tract fibers on contralateral hindlimb motoneurons.A, Monosynaptic connections via crossing axon collaterals of RF neurons (1, 2) with cell bodies located either on the opposite or same side as motoneurons and of RF neurons descending at the side of location of motoneurons (3); effects of the latter were eliminated by a hemisection of the spinal cord a few segments rostral to the motoneurons. B, Hypothetical disynaptic connections between reticulospinal tract neurons and contralateral motoneurons via interneurons of the lumbosacral enlargement: commissural neurons (4) and interneurons located at the same side as the motoneurons, the latter activated either by crossing collaterals of the uncrossed reticulospinal tract fibers (5) or by collaterals of the crossed reticulospinal tract fibers (6); effects of the latter were eliminated by a hemisection. C. Alternative disynaptic connections via more rostrally located neurons: long propriospinal tract neurons (7) and other indirectly activated reticulospinal neurons (8) and their crossing segmental axon collaterals. Reticulospinal neurons labeled A,D, and G might represent the same RF neurons. Effects mediated by connections 1,4, and 8 might thus be evoked in parallel. The same may be true for effects mediated by connections2 and 4, if reticulospinal neurons labeled B and F represented the same neurons. Interneurons located on the same side as motoneurons might be excited not only by connections 5 and 6but also by axon collaterals of other reticulospinal neurons and propriospinal neurons; however, these possibilities are not indicated in the diagrams for the sake of simplicity. Any additional synaptic actions of propriospinal neurons or indirectly activated reticulospinal tract neurons mediated by spinal interneurons would, however, be evoked trisynaptically. Int, Interneurons; Com, commissural neurons; PN, long propriospinal neurons;Mn, motoneurons; i, ipsilateral;co, contralateral. Arrows indicate sites of stimulation.
Placement of electrodes in the reticular formation and histological verifications. Electrodes were inserted through the cerebellum at an angle of 30° (tip directed rostrally). The initial target position was the lateral border of the medial longitudinal fasciculus (MLF) at Horsley–Clarke coordinates posterior 9–10, lateral 1.0, and horizontal −5 but in some experiments in nucleus reticularis gigantocellularis, 1–1.5 mm more lateral. The final position was adjusted on the basis of records of descending volleys from the surface of the lateral funiculus at a T11–T13 level. In most experiments, the electrodes were placed at the side opposite to the side of location of motoneurons recorded from, but in two experiments, they were placed bilaterally. The electrodes were left at sites from which distinct descending volleys were evoked by single stimuli at a latency of 2.0–2.2 msec at a threshold of 20–50 μA. These sites were marked at the end of the experiments with an electrolytic lesion and were verified on 100-μm-thick frontal sections of the brainstem. These were cut in the plane of insertion of the electrodes using either a vibratome or a freezing microtome and were counterstained with cresyl violet. The distribution of the stimulation sites is indicated in Figure 10, C andD.
Fig. 10.

Stimulation sites in the medulla. A, B, Photomicrographs in the planes of the electrode tracks indicated by the dashed lines from the experiments in which the records illustrated in Figure 9A–D were obtained; the electrolytic lesions were made at H coordinates 6 and 5.5. C, Stimulation sites from which excitation or inhibition of commissural neurons or both were evoked at stimulus intensities ≤100 μA; all these sites were ipsilateral.interneur., Interneurons. D, Stimulation sites from which disynaptic EPSPs, IPSPs, or both were evoked in motoneurons (motoneur.) at stimulus intensities ≤100 μA; those at the left side (ipsilateral) were used to compare effects of stimuli applied at the right side (contralateral). Upward and downward triangles indicate stimulation sites in the same experiment.
Stimulation. The reticular formation was stimulated monopolarly, using a 0.5 mm electrolytically etched tungsten wire insulated except for its tip as a cathode and a wire inserted into a neck muscle as an anode. Constant-current single, double, or triple stimuli 5.0 msec apart (0.2 msec, 50–200 μA) were used. Reticulospinal tract fibers were also stimulated (0.2 msec, 200–500 μA) at a lower thoracic or upper lumbar levels using two silver ball electrodes in contact with the lateral funiculus (contralaterally with respect to the motoneurons recorded from), but in this case, together with any other descending tract fibers (e.g., vestibulospinal and propriospinal), running in the lateral funiculus. Peripheral nerves were stimulated with constant-voltage stimuli (0.1 msec, intensity expressed in multiples of threshold for the most sensitive fibers in a given nerve).
Recording and analysis. Intracellular records from motoneurons and extracellular records of field potentials in motor nuclei were made using glass micropipettes (1.5–2.0 μm tip diameter) filled with a 2 m potassium citrate solution. Extracellular records from commissural neurons likely to mediate PSPs recorded in motoneurons were made with glass micropipettes filled with a 2m sodium chloride solution (2.0–2.5 μm tip diameter) or a 4% solution of rhodamine dextran in 0.9% sodium chloride (1.5–2.0 μm tip diameter). The rhodamine dextran-filled micropipettes were used in experiments in which commissural neurons were subsequently penetrated and intracellularly labeled (B. A. Bannatyne, D. J. Maxwell, S. E. Edgley, I. Hammar, and E. Jankowska, unpublished data). Records of incoming afferent and descending volleys associated with the PSPs or spike potentials of the motoneurons or commissural neurons were taken from the surface of the spinal cord with a silver ball electrode in contact with the dorsal columns close to the dorsal roots entry zone or the lateral funiculus on the side of location of the motoneurons, unless stated otherwise, usually within 5–10 mm of the microelectrode recording site. DC recording or low-pass filters of 1 Hz were used when recording from motoneurons, and both the original records and averages of 10 or 20 single-sweep records were stored (using a software designed by E. Eide, N. Pihlgren, and T. Holmström, Department of Physiology, Göteborg University). The measurements of amplitudes and latencies of PSPs evoked from the reticular formation were made from the averaged records. The measurements of latencies of responses of interneurons were made from single-sweep records. Paired Student's ttest was used for the statistical analysis. The reproduction of the records was made using CorelDraw 8 system.
Sampling. Effects of RF stimulation were analyzed in a sample of 140 motoneurons located in lumbar fourth, fifth, sixth, and seventh segments (see Table 1, columns 1 and 2). Most of these motoneurons (n = 87) had action potentials of 50–80 mV, membrane potential of 50–70 mV, or both. Motoneurons with action potential amplitudes of 35–50 mV (n = 53) were included only when the recording was stable.
Table 1.
Motoneurons in which disynaptic EPSPs, IPSPs, or both were evoked by trains of three stimuli
| Motoneuron | n | EPSPs | IPSPs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Third 100 μA | Third 200 μA | Third 100 μA (mV) | Third 200 μA (mV) | Latency (disynaptic) (msec) | Third 100 or 200 μA | After EPSP (n) | Without EPSP (n) | Latency (msec) | |||||||||
| n | % | n | % | Stimulus | Early | Late | n | % | Stimulus | Early | Late | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| GS | 26 | 17 | 65 | 26 | 100 | 0.54 | 0.82 | 4.38 | 1.39 | 0.46 | 7 | 27 | 6 | 1 | 4.88 | 1.84 | 0.88 |
| Q | 20 | 17 | 85 | 20 | 100 | 0.6 | 0.82 | 4.03 | 1.37 | 0.59 | 9 | 45 | 8 | 1 | 4.45 | 1.49 | 0.66 |
| PBST | 20 | 11 | 55 | 20 | 100 | 0.5 | 0.65 | 4.51 | 1.5 | 0.55 | 1 | 5 | 1 | 0 | 5.18 | 2.11 | 1.09 |
| FDL | 14 | 12 | 86 | 14 | 100 | 0.8 | 1.29 | 4.47 | 1.48 | 0.54 | 2 | 14 | 1 | 1 | 5.12 | 1.79 | 0.55 |
| Tib | 33 | 14 | 42 | 18 | 55 | 0.59 | 0.7 | 4.66 | 1.42 | 0.53 | 9 | 27 | 3 | 6 | 4.55 | 1.41 | 0.63 |
| DP | 15 | 5 | 33 | 5 | 33 | 0.25 | 0.31 | 4.61 | 1.36 | 0.54 | 1 | 7 | 0 | 1 | 4.48 | 1.47 | 0.45 |
| Sart | 8 | 1 | 13 | 1 | 13 | 0.26 | 0.29 | 4.12 | 1.36 | 0.56 | 8 | 100 | 1 | 7 | 4.12 | 1.38 | 0.6 |
| Other | 4 | 3 | 75 | 4 | 100 | 0.39 | 0.54 | 4.22 | 1.35 | 0.53 | 0 | 0 | 0 | 0 | |||
| Total | 140 | 80 | 57 | 1081-160 | 76 | 0.58 | 0.79* | 4.38 | 1.42 | 0.53 | 37 | 26 | 20 | 17 | 4.52* | 1.53* | 0.681-160 |
| 0.04 | 0.06 | 0.04 | 0.03 | 0.02 | 0.06 | 0.04 | 0.03 | ||||||||||
Columns 1, 2, Total number of motoneurons of different species sampled. Columns 3, 4, Numbers and percentages of motoneurons in which EPSPs were evoked after the third 100 μA stimulus. Columns 5, 6, Numbers and percentages of motoneurons in which EPSPs were evoked after the third 200 μA stimulus. Columns 7, 8, Mean amplitudes of EPSPs evoked by the third 100 and 200 mA stimuli. Columns 9–11, Latencies of EPSPs evoked disynaptically by either the second or third 100 μA stimuli from the stimulus artifacts from the first and second components of the descending volleys. Columns 12, 13, Numbers and percentages of motoneurons in which IPSPs were detected after the third stimulus at 100 or 200 μA. Columns 14, 15, Numbers of motoneurons in which the IPSPs were or were not preceded by EPSPs. Columns 16–18, Latencies of IPSPs evoked by the second or third stimulus (100 or 200 μA) from the stimulus artifacts from the first and second components of the descending volleys. In the bottom rows of columns 7–11 and 16–18 are means and SEM for all motoneurons.
,
F1-160: Statistically significant differences for the data in bold are indicated by asterisks (* = 0.01-0.05; **p < 0.001, probability of null hypothesis). These were between effects of 100 and 200 μA stimuli in columns 3 and 5 and 7 and 8 and for latencies of EPSPs and IPSPs in the whole sample (in columns 9–11, 16–18). However, differences between latencies of EPSPs and IPSPs were significant for those evoked in Q and Sart motoneurons (pooled data) but not for those evoked in Tib and G-S motoneurons.
The sample of commissural neurons included 24 extracellularly recorded neurons located in the ventral horn of the L4–L5 segments, at the sites at which largest monosynaptic focal field potentials were evoked by single RF stimuli, ipsilaterally to the RF stimulation side. All of these were monosynaptically excited after RF stimuli and were antidromically activated from contralateral GS motor nuclei in the L7 segment. A collision between responses that were evoked synaptically and those evoked by stimuli applied in the motor nuclei (most often <50 μA) was used to verify the antidromic activation. Responses were classified as evoked monosynaptically when they were induced at latencies not exceeding 1 msec from the initial component of the descending volleys after RF stimuli. Whenever tested (in 12 neurons), stimuli applied at the thoracic level failed to induce antidromic activation of neurons projecting to the L7 contralateral motor nuclei, extending observations on >70 previously investigated L4–L5 and L6 commissural neurons with input from muscle afferents (Harrison et al., 1986; Jankowska and Noga, 1990). The same thoracic (Th) stimuli (0.2 msec, 0.2–1 mA, applied by pairs of electrodes in contact with the left and right lateral funiculi) were effective in inducing antidromic activation of other, most likely spinocerebellar tract, neurons with group I input that were not antidromically activated from the motor nuclei in the same experiments.
Results
Many different potential routes through which reticulospinal tract neurons can influence contralateral hindlimb motoneurons exist, and the major ones are indicated in Figure 1. Monosynaptic EPSPs could be mediated by ipsilaterally descending reticulospinal neurons, via crossed axon collaterals terminating in the ventral horn (Nyberg-Hansen, 1965; Kausz, 1991; Matsuyama et al., 1999), as indicated by connections labeled 1 and2 in Figure 1A. Monosynaptic EPSPs could also be evoked by contralaterally descending reticulospinal neurons (Nyberg-Hansen, 1965; Mitani et al., 1988b,c; Matsuyama et al., 1993) and their local axon collaterals (Fig. 1A,3). On the basis of morphological studies (Nyberg-Hansen, 1965; Holstege et al., 1979; Matsuyama et al., 1999), the number of contacts between reticulospinal neurons and contralateral motoneurons was expected to be smaller than with ipsilateral motoneurons but not negligible.
Disynaptic EPSPs or IPSPs could be induced in several ways. They might, for example, be mediated by lamina VIII commissural neurons, as indicated by connection 4 in Figure 1B. Two alternative pathways via spinal interneurons located at the same side as α-motoneurons are indicated by connections 5 and6 in Figure 1B. These interneurons could be activated via crossing axon collaterals (5) of the uncrossed reticulospinal tract fibers or via the crossed reticulospinal tract fibers (6). Two other alternative disynaptic pathways are indicated in Figure 1C. These would involve more rostrally located propriospinal neurons (connection7) and other indirectly activated reticulospinal tract neurons (connection 8).
Mass records of volleys set up by reticulospinal stimulation
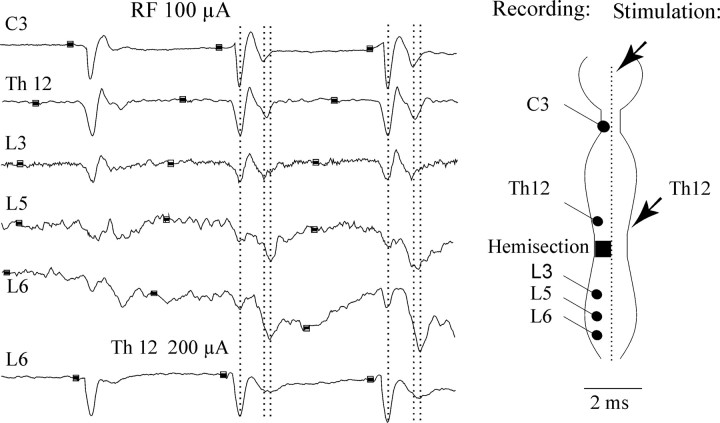
As indicated above and in Figure 1, the crossed actions evoked by the RF stimuli could be relayed at both spinal and supraspinal levels and by various kinds of neurons at each level. Some clues as to the relative importance of these might be gained from mass records of activity evoked by RF stimulation, so in initial observations, we examined the descending volleys evoked by RF stimulation. In preparations with the spinal cord intact, stimulation of RF axons is followed by a fast synchronous volley recorded through most of its length and somewhat later volleys, particularly caudally (Shapovalov, 1969; Floeter et al., 1993). The initial volley remains constant to each stimulus of a train of RF stimuli, whereas the later volleys show marked temporal facilitation and post-tetanic potentiation. A consistent observation from all of our experiments was that similar late volleys were evoked below the level of the spinal hemisection made within the L2 segment. Figure2 illustrates volleys recorded at cervical and thoracic levels above the hemisection and at several lumbar levels below the hemisection from one experiment. Some temporal facilitation of a second component occurred supraspinally, as can be seen from the recordings at a cervical level. However, considerable additional temporal facilitation occurred at a spinal level, particularly below the L3 segment. The relative amplitude of the second components after the third stimulus was approximately half that of the first components at the cervical and thoracic levels (C3 and T12), but the two components were approximately the same size at the upper lumbar level (L3), and the second components were more than three times larger than the first components at the lower lumbar levels (L5–L7). Examples of similar relationships between the two components of the descending volleys at a lower lumbar level in other experiments are shown in Figures 3, 5-7, and 9. Other features of the second components of the descending volleys illustrated in Figure 2are that they are substantially broader at lumbar than at cervical and thoracic levels and that the onsets of both the positive and the following negative peaks of the second components are delayed at the lower lumbar level. In this experiment the delay was 0.2–0.3 msec. Similar delays were seen in other experiments, but configurations of the descending volleys in different experiments made them difficult to be quantified in a reliable way. The broadening and delays in the peak of the second components could be explained by an increasing degree of recruitment of somewhat slower-conducting spinal interneurons.
Fig. 2.
Temporal facilitation of the second components of descending volleys induced by RF stimuli. Fromtop to bottom are records from the surface of the left lateral funiculus at the indicated levels. All these records are from the same experiment and illustrate descending volleys evoked by stimuli applied in the right MLF and, for comparison, to the right lateral funiculus at T12 (Th12). In thediagram, the stimulation sites are indicated byarrows, and the recording sites are indicated byfilled circles. Amplitudes of the first positive (downward) components at C3 and Th and at L3, L5, and L6 were normalized to aid the comparison of the second components. The records are aligned so that the peaks of the first components of the descending volleys coincide. Shock artifacts are removed, and thick segments of the lines indicate the times of application of the stimuli. The three dashed linescoincide with the peaks of the first components and peaks of the second components at C3 and L6.
Fig. 3.
Examples of PSPs evoked in a contralateral GS α motoneuron by RF stimulation. A–C, EPSPs evoked by triple RF stimuli at 60, 70, and 100 μA and corresponding records of descending volleys (top, bottom traces, respectively). D, Extracellular field potentials recorded just outside the motoneuron; note that they displayed a similar temporal facilitation. The arrowindicates the terminal potential. E–G, Effects of single, double, and triple RF stimuli at 100 μA. The gray trace overlying the third EPSP inG is the one from E, normalized to the size of the EPSP evoked the third stimulus. Note the faster decline of the EPSP evoked by the third stimulus, indicating that IPSPs were also evoked. H, Expanded view of the middle part (indicated in F by the dotted horizontal line) of the records in F, with extracellular records of field potentials being subtracted from the intracellularly evoked EPSP to allow a better estimate of EPSP onset. Dotted vertical lines indicate the positive peaks of the first and second components of the descending reticulospinal volley and the onset of the EPSP. Voltage calibration in C is forA–C and E–G. In this and the following figures, the negativity is downward in microelectrode recordings (intracellular and extracellular, usually top traces) and upward in records from the surface of the spinal cord (usually bottom traces).
Fig. 5.
Examples of EPSPs likely to be evoked monosynaptically in three motoneurons. Top traces are intracellular records from a motoneuron, and bottom traces are from the surface of the spinal cord. Dotted lines indicate the positive peaks of the first component of the descending volley and the onset of the presumably monosynaptically and disynaptically evoked EPSPs. A–C, EPSPs evoked in a DP motoneuron by stimuli applied in the RF (A), at a thoracic level (B), and by group I afferents (C); in C, the last EPSP fromA is superimposed (gray) on the group Ia EPSP after its amplitude has been normalized.D–F, EPSPs evoked in an FDL motoneuron by RF stimuli at two intensities as indicated. Note that the first two weaker stimuli and the first stronger stimulus evoked a short latency EPSP, whereas the following stimuli also evoked a later EPSP. F, First EPSP in D shown expanded. G, H, EPSPs evoked in a PBST motoneuron by stimuli applied at two different depths along the electrode track shown in Figure 10A. Note that EPSPs evoked from the more dorsal site followed each of the three stimuli and appeared at a shorter latency, whereas those evoked from the more ventral site were induced only by the second and third stimuli and at a longer latency (at the level of the third dotted line). I, Extracellular (Extracell.) field potentials evoked by the same stimuli as the records in H: truncated shock artifacts.
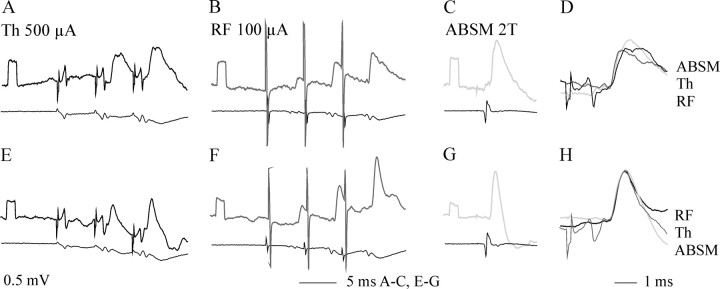
Fig. 6.
Temporal facilitation of EPSPs evoked from the Th level. A–E, H, Records from two Q motoneurons.A, E, EPSPs evoked by Th stimuli. B, F, EPSPs evoked from the RF. C, G, Monosynaptic EPSPs evoked by group Ia afferents in an ABSM nerve. D, H, Superimposed EPSPs of all three origins, expanded and normalized to the same initial peak amplitude. Note the similar rise time.
Fig. 7.
Temporal facilitation of IPSPs.A–D, Examples of IPSPs evoked by RF stimuli in four Q motoneurons (all from the same experiment, along the electrode track illustrated in Fig. 10B). In A–C, the gray traces superimposed on the last PSPs are those evoked by the preceding stimulus to show the faster decline of the last EPSPs, which is an indication that they were followed by IPSPs growing in size after successive stimuli. The superimposed EPSP was normalized in A but at the original size in B andC. D, Records of IPSPs apparently not associated with EPSPs. The superimposed gray trace is that of an IPSP evoked by Q group I afferents in an unidentified flexor motoneuron recorded in the same segment. The amplitude of the latter was normalized to that of the IPSP evoked from the RF to allow a better comparison of their time course. E–H, Records from a Tib motoneuron recorded in another experiment: E, just after penetration of the motoneuron; F, after its depolarization by 20 nA; G, after removal of the polarization current; H, after its hyperpolarization by 10 nA. Note an increase and a much clearer onset of the IPSP (indicated by the dotted line) after the depolarization.
Fig. 9.
Extent of the areas from which disynaptic PSPs were evoked in contralateral motoneurons. A, B, Intracellular records from a GS motoneuron (moton.) showing effects of stimuli (70 μA) applied along the electrode track indicated in Figure 10A, dotted line, and descending (Desc.) volleys evoked by the same stimuli. C, D, Similar series of records from a Sart motoneuron and the descending volleys induced by stimuli (80 μA) applied along the electrode track indicated in Figure10B, dotted line. Double dotted lines in B and D coincide with the first and second components of the descending volleys.
Because the volleys recorded from the surface of the spinal cord may reflect neuronal activity from a large area, these observations do not associate the RF volleys with any specific neuronal pathways. The early component of the volleys recorded below the level of the hemisection could reflect action potentials in the ipsilateral reticulospinal tract fibers as well as in their local crossed axon collaterals (Fig. 1A). The large later volleys could reflect action potentials in axons of either lumbar commissural neurons or interneurons excited by crossed RF axon collaterals (Fig.1B). Attempts to localize the sources of these volleys by using intraspinal recording with a tungsten electrode at different sites within the white and gray matter were generally unsuccessful, because similar volleys were widespread. However, the monosynaptically excited interneurons were encountered only ipsilaterally.
Figure 2, bottom traces, shows that volleys evoked by stimulation of the spinal lateral funiculus at the T12 level also have late components that show temporal facilitation. These might reflect activation of a variety of fibers but should also include the fibers that contribute to the second components of the descending volleys after RF stimuli.
Taken alone, recordings of the volleys cannot be used to determine pathways to motoneurons, but these observations become more important in relation to the recordings of PSPs evoked by RF stimulation.
Disynaptically evoked EPSPs
The most frequently seen effects of RF stimulation on contralateral motoneurons were EPSPs with properties indicative of a disynaptic relay, because they showed marked temporal facilitation and appeared at segmental latencies 1.3–1.5 msec from the earliest components of the descending volleys. Such EPSPs were found in 76% of the motoneurons when stimuli of 200 μA were used.
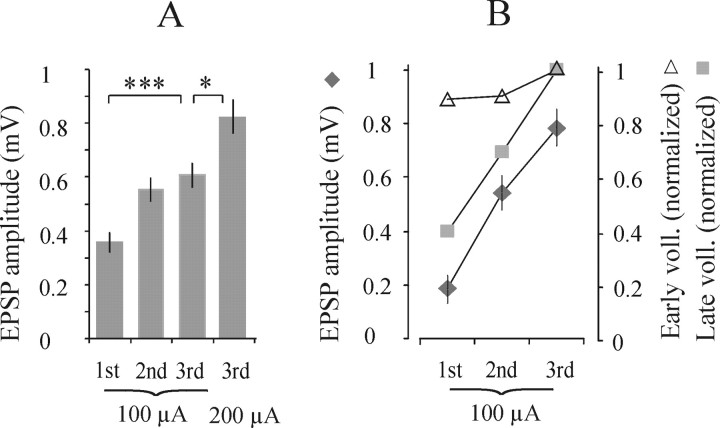
Temporal facilitation
Figure 3A shows that when three relatively weak stimuli were used, the first was ineffective, and the EPSPs appeared only after the second or third stimulus. When the stimulus intensity was increased, all three stimuli were often followed by EPSPs, but those evoked by the second or third stimulus or both were larger (Fig.3) and appeared at 0.1–0.2 msec shorter latencies. Mean amplitudes of EPSPs evoked by successive stimuli are shown in Figure4A. Temporal facilitation was also found to be marked on extracellular field potentials recorded in motor nuclei, as exemplified in Figure3D. After the first stimulus, these potentials were either absent or very small; they became more distinct after successive stimuli. They were found in all of the motor nuclei explored. The marked temporal facilitation of many EPSPs evoked from the reticular formation, in particular when they only appeared after the second or third stimulus, argues against the possibility that these EPSPs were induced by direct actions of reticulospinal tract fibers on motoneurons (Fig. 1, connections 1, 2) and indicates disynaptic coupling (Fig. 1, connections 4, 5, 7, 8).
Fig. 4.
Amplitudes of EPSPs evoked from RF.A, Mean amplitudes ± SEM of EPSPs evoked by the first, second, and third stimuli at 100 μA and by the third stimulus at 200 μA. B, Relationships between amplitudes of EPSPs evoked by 100 μA and amplitudes of early and late components of the descending volleys (voll.) evoked by successive stimuli (with respect to those evoked by the third stimulus).B includes data for EPSPs with peak amplitudes exceeding 0.5 mV, which were evoked by the third stimulus in 28 motoneurons in the L7 segment in three experiments (GS, PBST, and FDL). Differences between amplitudes of EPSPs evoked by the third 100 and 200 μA stimuli and between effects of the third and first (but not the second) stimuli are highly statistically significant.
Latencies of temporally facilitated EPSPs evoked from the reticular formation and the relationship between these EPSPs and the descending volleys
The EPSPs described above were evoked at a mean latency of 4.38 ± 0.04 msec from the second or third stimulus of a train of three (for details, see Table 1, column 9). The latencies of the EPSPs were also measured with respect to the descending volleys after RF stimuli, recorded from the spinal surface close to the motoneuron recording site. The mean latency of these EPSPs was 1.42 ± 0.03 msec, measured from the positive peak of the earliest components of the descending volleys recorded within the same segment (Table 1, column 10). Even when the EPSPs were evoked by single stimuli, they were thus too long to be attributable to direct actions of the fastest conducting reticulospinal tract fibers. On the other hand, the EPSPs had latencies averaging 0.53 ± 0.02 msec from the positive peak of the second component of the descending volleys (Table1, column 11), which is compatible with monosynaptic coupling between fibers responsible for these second components and the motoneurons. The time relationships between the EPSPs and the two components of the descending volleys are illustrated in the expanded records of Figure3H, where the first two dotted lines coincide with the first and second positive peaks of the volleys, and thethird dotted line coincides with the onset of the EPSP. Terminal potentials (Munson et al., 1980), which preceded the EPSPs recorded in individual motoneurons and the field potentials by ∼0.3 msec (Fig. 3D, arrow) appeared at latencies 1.12 ± 0.02 and 0.22 ± 0.04 msec from the first and second components of the descending volleys, respectively (measurements for the 20 most distinct potentials seen).
The amplitudes of the EPSPs appeared not to be related to the amplitudes of the first components of the descending volleys, which were almost constant (Fig. 4B, triangles), whereas only the second and third stimuli were often effective. In contrast, a close relationship has been found between amplitudes of the EPSPs and those of the second components of the descending volleys. The relationship is summarized in Figure 4B.
The records of disynaptic EPSPs evoked by RF stimuli in Figures5C and 6, D andH, also show that their time profiles resembled those of oligosynaptic EPSPs of group I origin recorded in the same motoneurons. The time to peak was measured for 23 EPSPs with amplitudes >0.5 mV, which were induced by the second RF stimulus of 100 μA and which did not appear to be cut short by the following IPSPs. The time to peak was 0.86 ± 0.06 msec (range, 0.56–1.54 msec).
Monosynaptically evoked EPSPs
Some reticulospinal neurons have axon collaterals given off at a spinal level that cross the ventral commissure and reach the ventrolateral part of the ventral horn (Matsuyama et al., 1993, 1999). Such collaterals might thus induce monosynaptic EPSPs in contralateral motoneurons (Fig. 1A, connections 1, 2). Two kinds of data from our experiments show monosynaptically evoked actions of reticulospinal neurons on some contralateral motoneurons.
First, in some motoneurons, EPSPs were induced by the first as well as by the second and third stimuli at latencies of 1 msec or less from the early components of the reticulospinal descending volleys. Second, these same EPSPs showed negligible temporal facilitation after successive stimuli (examples shown in Fig. 5A,D,E). The onsets of these EPSPs either preceded or coincided with the positive peak of the late components of the descending volleys (Fig.5F, top, bottom records) and with the onset of the field potentials (Fig. 5G,I, top records). However, after the second and third stimuli, additional later PSPs, which did show marked temporal facilitation, were often superimposed on these EPSPs. In Figure 5, D andE, the onset of the additional components is indicated bydotted lines coinciding with the inflections in the rising or declining phases of the EPSPs evoked by the first stimulus. The short- and longer-latency EPSPs were sometimes evoked from different sites along an electrode track (Fig. 5, compare G, H).
EPSPs with these properties could be classified as monosynaptic and were found in 14 motoneurons (5 PBST, 2 GS, 3 FDL, and 4 DP) in four different experiments. They were induced at current intensities as low as 50 or even 20 μA and ranged in amplitude between 0.1 and 0.4 mV.
EPSPs evoked by stimuli applied to the contralateral lateral funiculus at a low thoracic level
The effects of stimuli applied within the RF were usually matched by effects of stimuli applied to the contralateral lateral funiculus of the spinal cord above the level of the ipsilateral hemisection, as would be expected for effects evoked by the same fibers stimulated in the spinal cord. In most motoneurons, EPSPs evoked by Th stimuli displayed features of disynaptically evoked EPSPs; their mean latencies from the initial component of the descending volley were 1.31 ± 0.02 msec, and they grew in parallel with the increases of the second components of the descending volleys and displayed temporal facilitation as potent as that of PSPs evoked from RF. The time courses of EPSPs evoked by Th stimuli usually resembled those of EPSPs evoked from the reticular formation, as exemplified in Figures 5, Aand B, and 6, D andH, but the amplitudes, the degree of the temporal facilitation, and the occurrence of additional later components depended on the stimulus intensities.
EPSPs classified as being evoked monosynaptically were evoked by Th stimuli in only 11 (12.5%) of 88 motoneurons tested. The latencies of these EPSPs were between 0.8 and 1 msec from the initial component of the descending volleys. However, even those with the shortest latencies showed some increase in amplitude after the second and third stimuli (as in Fig. 5B), but this may have been caused by an addition of later disynaptic components. Whether the monosynaptic EPSPs evoked by Th stimuli were evoked by reticulospinal or vestibulospinal or other fibers could not be decided, but in six motoneurons, the earliest components of the EPSPs were of amplitudes similar to those evoked from the reticular formation, with an example in Figure5B.
Disynaptically evoked IPSPs
IPSPs after RF stimuli (three stimuli at 100 or 200 μA) were detected in a smaller proportion of motoneurons than EPSPs (26%). However, this proportion (Table 1 column 13) is most likely an underestimate, because IPSPs were the only or the dominant effect of RF stimulation (as in records of Fig.7D) in a relatively small proportion of motoneurons (n = 17; Table 1, column 14). In others, they were superimposed on EPSPs and were therefore more difficult to detect. In these motoneurons, they were indicated by increasingly steep slopes of the declining phases of EPSPs after successive stimuli (Fig. 7A; n = 7) or by dips in the declining phases of EPSPs (Fig. 7B,C;n = 13). The presence of IPSPs became more marked when the motoneurons were depolarized, with an extreme case illustrated in Figure 7E–H. However, not all motoneurons were depolarized, and in those that were it was not always possible to attribute changes in the shape of the EPSPs to following IPSPs. IPSPs could therefore have been missed in a number of motoneurons.
Like the EPSPs, the IPSPs were rarely evoked by single stimuli, and they displayed marked temporal facilitation. The facilitation was evident whether or not the IPSPs were preceded by EPSPs. The latencies of the IPSPs were measured when their onset was distinct from the beginning or when it became distinct after 10–30 nA depolarization of the motoneurons. The estimated mean onset latency was 1.53 ± 0.04 msec from the first component of the descending volley and 0.68 ± 0.03 msec from the second component (Table 1, columns 17, 18). The segmental latencies of the IPSPs thus appear to be similar to the latencies of the EPSPs. The arguments for disynaptic coupling between reticulospinal fibers inducing the EPSPs and motoneurons therefore also apply to the IPSPs.
Distribution of EPSPs and IPSPs
Most of the motoneurons tested showed EPSPs with disynaptic characteristics, but they were not distributed in the same way in all motor nuclei. As shown in the Table 1, columns 3–6, they were evoked in most of the GS, Q, PBST, and FDL motoneurons by stimuli of 100 μA and in all of them by stimulation at 200 μA. However, they were found in only approximately half of Tib and an even smaller proportion of DP and Sart motoneurons recorded in the same experiments. Furthermore, the amplitudes of the EPSPs tended to match their distribution, being smallest in DP and Sart motoneurons (Table 1, columns 7, 8).
Monosynaptically evoked EPSPs were found in motor nuclei in which the proportion of disynaptically evoked EPSPs (PBST, FDL, and GS) was high as well as in those with a low proportion (DP). There does not appear to be any relationship between the proportions of motoneurons in which the EPSPs and IPSPs were found (Table 1, columns 6, 13). The only exception appears to be Sart motoneurons, with the largest proportion of IPSPs and the smallest proportion of EPSPs.
Evidence for the existence of commissural interneurons that might mediate disynaptic excitatory or inhibitory actions from the reticular formation on motoneurons
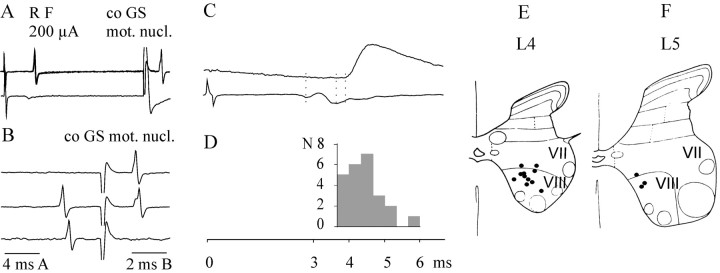
Given that RF stimuli evoke disynaptic PSPs in contralateral motoneurons and that a late component of the descending volleys increases considerably at recording sites progressively further caudally in the midlumbar segments of the spinal cord, spinal interneurons in pathways labeled 4 and 5 in Figure 1B should include commissural neurons located in the midlumbar segments. Recordings in these segments revealed that interneurons activated antidromically from the contralateral GS motor nuclei in the L7 segment and excited monosynaptically by ipsilateral fast conducting reticulospinal tract fibers are present in lamina VIII of the L3, L4, and rostral L5 segments. Examples of extracellular records from such neurons are shown in Figure8, A and B, and their locations are shown in Figure 8, E and F. The range of latencies at which these interneurons were excited from the RF was fairly narrow, 0.2–0.96 msec from the earliest components of the descending volleys recorded at the side of location of the interneurons. However, the conduction time along their axons varied considerably; the latencies of antidromic activation from the L7 segment ranged between 0.6 and 2.2 msec. The collision test showed that all were antidromically activated (as illustrated in Fig.8B). To verify that the delays of transmission through these neurons complied with the latencies of disynaptic EPSPs and IPSPs evoked in motoneurons, the following comparison has been made. The sums of latencies of synaptic activation by RF stimuli and of antidromic activation from motor nuclei were calculated for 24 extracellularly recorded interneurons, and these were related to the timing of RF actions on motoneurons. Figure 8D shows that these sums amounted to 3.65–4.35 msec for approximately half of the interneurons, i.e., corresponding to the period preceding the onset of the EPSPs evoked in motoneurons and overlapping with their rising phase. The shortest were only ∼0.7 msec shorter than the mean latencies of EPSPs and IPSPs induced in motoneurons (4.38 ± 0.04 and 4.52 ± 0.06 msec), an example of which at the same time base is shown in Figure 8C.
Fig. 8.
Lamina VIII commissural neurons antidromically activated from contralateral motor nuclei and monosynaptically excited from RF or MLF. A, Extracellular records from an interneuron (4 superimposed traces), illustrating highly synchronized short-latency responses after stimuli applied in the reticular formation and in the contralateral motor nucleus.B, Single-sweep records from the same interneuron showing that responses from the motor nucleus were prevented from appearing when synaptically evoked spikes preceded them at an interval of approximately twice the peripheral conduction time (the synaptic–antidromic collision test); the shock artifacts are truncated. D, Histogram of times of transmission through the commissural neurons (sums of latencies of the synaptic and antidromic activation) for 24 extracellularly recorded commissural interneurons, which were monosynaptically activated from the reticular formation. The histogram is in the same scale as the record of a disynaptic EPSP evoked in a motoneuron in C (expanded record from Fig. 2H). E, F, Location of 16 intracellularly labeled interneurons of the present sample in the L4 and L5 segments. They were injected with rhodamine dextran and examined under confocal microscopy. The location of these neurons is indicated on diagrams of the gray matter with the borders between Rexed's laminas (Rexed, 1954) indicated.
Origin of oligosynaptic PSPs evoked from the reticular formation
Stimuli applied in any of the reticular nuclei might activate, directly or indirectly, neurons located at different distances from the stimulation sites. No attempt has therefore been made to define the location of reticulospinal neurons that evoked the oligosynaptic EPSPs and IPSPs described above. However, as judged by the intensity of the stimuli needed to induce these PSPs, the most effective were stimuli applied within or at the lateral border of the MLF (0.5–1 mm from the midline). The thresholds for evoking these PSPs were 20–60 μA and were similar to the thresholds of the descending volleys. Stimuli applied at sites within the nucleus reticularis magnocellularis, 2–2.5 mm from the midline, evoked similar effects but only at higher intensities (at thresholds of 100 or even >200 μA), and these effects were induced after only the third, rather than the second or first, stimulus. Considering the lower effectiveness of stimuli applied at these locations and the risk for spread of current of stronger stimuli to vestibulospinal tract fibers, the data from the 27 additional motoneurons recorded in these experiments have not been included in the results presented above.
When effects of stimuli of 60–80 μA applied at different depths were compared, the most effective areas were found to extend vertically over ∼1 mm (Fig.9A,C) and to correspond to the middle and ventral parts of the MLF (Fig.10A,B), the same parts from which the largest descending volleys were evoked (Fig.9B,D) and corresponding to the areas from which short-latency EPSPs were evoked in ipsilateral motoneurons in a previous study (Floeter et al., 1993). Much smaller effects were evoked from sites 0.5 mm above or below this area, and stimuli of 60–80 μA became ineffective 1–1.5 mm above or below the optimal sites.
The weaker effects from the lateral parts of the reticular formation and from the area ventral to the MLF may be used as an argument for the mediation of the crossed oligosynaptic synaptic actions described here by the reticulospinal fibers rather than by other fibers stimulated within the reticular formation.
A comparison of effects evoked from the left and right sides was attempted to provide an indication as to whether the left-side reticulospinal tract neurons with crossed axons (Fig.1B, cell B, which could be stimulated on both the right and left sides) contribute to the PSPs evoked in the left-side motoneurons. However, the results were inconclusive. When thresholds of EPSPs or IPSPs evoked by stimuli on the left side exceeded 150–200 μA, the spread of current to reticulospinal tract neurons on the right side could not be excluded, because such strong stimuli may excite fibers within a 1–2 mm radius (Gustafsson and Jankowska, 1976). With stimuli of 100 μA or less, the spread of current to the right MLF would be less likely (but still possible). In two such experiments, stimuli applied on the left side (Fig.10D, triangles) and on the right side were found to evoke similar disynaptic PSPs (EPSPs or IPSPs), although PSPs evoked from the left side were smaller and appeared at longer latencies. In 25 motoneurons, stimuli (100 μA) applied at similar distances from the midline contralaterally and ipsilaterally (Fig.10D, black arrows) evoked EPSPs of 0.62 ± 0.07 mV at latencies of 4.04 ± 0.03 msec and 0.31 ± 0.05 mV at latencies of 4.14 ± 0.03 msec, respectively. The differences between both of these values were statistically significant. Similar differences were seen in another experiment in which the distance from the midline was greater ipsilaterally than contralaterally (open arrowheads). However, whether lower-amplitude and longer-latency PSPs evoked from the left side were caused by a smaller number of fibers stimulated at this side or a smaller number of fibers activated by spread of current to the right side could not be established.
Discussion
The first two questions of this study were whether reticulospinal tract neurons induce monosynaptically and disynaptically evoked PSPs in contralateral motoneurons, and our results give unequivocal answers to these questions. They show that short-latency EPSPs and IPSPs are evoked in most contralateral motoneurons after stimuli applied within the RF and MLF. The properties of most of these PSPs indicated that disynaptic EPSPs and IPSPs were evoked in most motoneurons, with monosynaptic EPSPs in a small proportion of motoneurons.
The third question concerned neurons mediating the crossed disynaptic actions of reticulospinal tract neurons. This question may at present be only partially answered. Our results show that these actions are likely to be mediated predominantly by interneurons in the lumbosacral enlargement and that commissural neurons in Rexed's lamina VIII in midlumbar segments have the required properties. However, we cannot exclude a contribution of other neurons.
How reliably may EPSPs and IPSPs evoked from RF in contralateral motoneurons be classified as evoked monosynaptically or disynaptically by fast-conducting RF fibers?
The differentiation between monosynaptically and disynaptically evoked PSPs on the basis of latencies is not always reliable, especially when synaptic actions are evoked by both fast- and slow-conducting fibers. This factor was not of major consequence for ipsilateral actions of reticulospinal fibers in view of the high conduction velocities of these fibers (90–140 m/sec; Shapovalov, 1969;Grillner et al., 1971; Eccles et al., 1975) and because their axon collaterals terminate within fairly restricted areas (Matsuyama et al., 1997). Crossing axon collaterals of reticulospinal tract fibers (Fig.1A, connections 1, 2) extend over a much longer distance (Matsuyama et al., 1988; Matsuyama et al., 1997). An additional delay of up to 1 msec in effects mediated by these collaterals might thus occur. However, the segmental delays of EPSPs fulfilling criteria of monosynaptic EPSPs (0.8–1.05 msec from the first component of the descending volleys) indicate that at least some of the crossing axon collaterals have a relatively high conduction velocity. A disynaptic linkage between similarly fast-conducting reticulospinal fibers and contralateral motoneurons would thus require only marginally longer latencies (the mean was 1.42 ± 0.53 msec). However, the temporal facilitation of the longer-latency EPSPs and IPSPs and the need for two or three stimuli to evoke them provide the strongest indication of disynaptic coupling.
The similar time course of PSPs evoked by RF and thoracic stimuli and by group I afferents (Figs. 5C, 6D,H,7D) suggests in addition that crossed monosynaptic EPSPs and disynaptic EPSPs and IPSPs of reticular origin are induced by presynaptic volleys that are as synchronous as afferent volleys in group Ia afferents (i.e., very synchronous). The times to peak of RF EPSPs (0.86 ± 0.06 msec; range, 0.56–1.54 msec), for example, were within ranges for EPSPs evoked by Ia afferents (0.86–2.9 msec;Burke, 1967) and for disynaptically evoked Ia IPSPs (0.75–1.2 msec;Jankowska and Roberts, 1972).
Stimuli applied within the reticular formation or even in the MLF might activate a number of other fibers in addition to those of the reticulospinal tract fibers. These might include spinoreticular tract fibers and collaterals of the corticospinal tract fibers, which provide input to reticulospinal neurons. However, there are at present no indications that ascending or descending tract neurons projecting to the reticular formation synapse with contralateral motoneurons or induce disynaptic excitation or inhibition, and 100 μA or weaker stimuli would be too weak to consider the spread of current to the vestibulospinal tract fibers that could induce such effects.
Indications for a spinal relay of the indirect RF synaptic actions
Our observations show that disynaptic actions of RF stimuli in the contralateral motor nuclei are linked to the second (indirect) components of the descending volleys. They also indicate a major contribution of neurons of the lumbosacral enlargement to these second components, because, as illustrated in Figure 2, size relationships between the first and second components of the descending volleys dramatically changed within the lumbosacral enlargement where the first component decreased (most likely because of a decreasing number of reticulospinal fibers projecting caudal to thoracic segments), whereas the relative size of the second component increased. These size relationships are taken to indicate that the earlier and later volleys reflect activity in different fibers, the earlier in directly activated reticulospinal axons and the later in axons of other indirectly activated neurons, and that the contribution of interneurons located within the lumbosacral enlargement becomes at this level much more important for the relayed actions of reticulospinal neurons than the contribution of more rostrally located neurons.
The temporal facilitation seen at the cervical level may be attributed to the increasing effectiveness of the trans-synaptic excitation of reticulospinal tract neurons by successive stimuli (as previously found by Ito and McCarley, 1987; McCarley et al., 1987). Increases at the thoracic level might also be attributed to the increasing effectiveness of excitation of long propriospinal neurons. The much more potent temporal facilitation at lumbar levels may be interpreted in two ways: either a larger proportion of indirectly (compared with directly) activated reticulospinal neurons project to the lumbosacral enlargement, or it could reflect the activity of lumbar interneurons recruited by the initial volley in the lumbosacral enlargement.
Because the second component of the descending volleys increased substantially in size between the L2 and L6 segments, one might hypothesize that neurons contributing to the second component are located primarily within the L3–L6 segments. However, the most decisive argument for a more important contribution of neurons located within the lumbosacral enlargement than more rostrally located neurons is the small proportion of neurons in which monosynaptic EPSPs were evoked by stimuli applied at a thoracic level. If indirectly activated reticulospinal tract neurons and rostrally located propriospinal neurons were primarily responsible for the second components of the disynaptic EPSPs evoked in contralateral motoneurons (as hypothesized in Fig. 1, connections 7, 8), this should be associated with a high frequency of monosynaptic effects of stimuli applied at a thoracic level to most rather than a few motoneurons. However, if Th stimuli primarily activated fibers synapsing with interneurons, the most frequently occurring PSPs evoked by Th stimuli should, as was found, have features of disynaptically rather than monosynaptically induced synaptic actions.
Interneurons with properties required of interneurons mediating crossed disynaptic actions evoked from the reticular formation have been found in Rexed's lamina VIII. These are interneurons that are powerfully monosynaptically excited by RF stimuli and that project to contralateral motor nuclei (Fig. 1B, connection4). The correspondence between the latencies of disynaptic RF actions and the sums of latencies of monosynaptically (from RF) and antidromically (from motor nuclei) evoked activation has been found to be good for a considerable proportion of these neurons. However, this cannot exclude the possibility that some disynaptic actions can be mediated by crossed axon collaterals of reticulospinal fibers and interneurons located at the same side as motoneurons (Fig.1B, connection 5) if the total conduction time along them is similar.
Activation of commissural neurons by reticulospinal neurons will be expected under all of the conditions when the reticulospinal tract neurons are activated, e.g., during locomotion induced by stimuli applied in the hook bundle of Russel or the cuneiform nuclei (Orlovsky, 1970; Shefchyk and Jordan, 1985; Jankowska and Noga, 1990; Mori et al., 1998). During locomotion in intact animals, reticulospinal neurons show phasic bursts (Drew et al., 1986). The commissural neurons will then be activated in parallel with activation of interneurons mediating disynaptic RF actions on ipsilateral motoneurons (Floeter et al., 1993;Gossard et al., 1996). Activation of commissural neurons would also be expected in scratching movements, which share a great part of the spinal machinery with locomotory movements (Burke, 1999; Perreault et al., 1999). They should also be activated in postural reactions.
A comparison of the distribution of RF actions on contralateral motoneurons found in the present study with those on ipsilateral motoneurons is made difficult by discrepancies in the descriptions of disynaptic RF actions on ipsilateral motoneurons. Those reported byGrillner et al. (1971) appeared to be evoked in a fairly restricted selection of motoneurons recorded from in anesthetized preparations. Of two other studies on unanesthetized decerebrate preparations, one led to the conclusion that disynaptic actions are evoked in most motoneurons of any motor nuclei (Floeter et al., 1993), whereas the other described only polysynaptic actions (Habaguchi et al., 2002).
Various motor patterns might be assisted by distinct populations of commissural neurons, some affecting contralateral motoneurons directly and others only indirectly. Even those synapsing with motoneurons might include a number of subpopulations, e.g., interneurons mediating crossed disynaptic excitation of either flexor or extensor motoneurons, or a bilateral increase in muscle tonus. Some of these might in addition assist in crossed excitation, and others might assist in crossed inhibition by group II muscle afferents (Aggelopoulos and Edgley, 1995; Aggelopoulos et al., 1996). Discussion of these points will therefore be postponed until more is known about properties of subpopulations of commissural neurons and relationships between them.
Footnotes
This work was supported by National Institutes of Health Grant NS 40 863. We thank Rauni Larsson for invaluable assistance during the experiments and for histological verifications.
Correspondence should be addressed to Elzbieta Jankowska, Department of Physiology, Medicinaregatan 11, Box 432, 405 30 Göteborg, Sweden. E-mail: elzbieta.jankowska@physiol.gu.se.
References
- 1.Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60–64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- 2.Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp Brain Res. 1990;80:83–95. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- 4.Burke RE. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967;30:1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- 5.Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- 6.Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol. 1990a;64:767–781. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- 7.Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J Neurophysiol. 1990b;64:782–795. doi: 10.1152/jn.1990.64.3.782. [DOI] [PubMed] [Google Scholar]
- 8.Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- 9.Eccles JC, Nicoll RA, Schwarz WF, Taborikova H, Willey TJ. Reticulospinal neurons with and without monosynaptic inputs from cerebellar nuclei. J Neurophysiol. 1975;38:513–530. doi: 10.1152/jn.1975.38.3.513. [DOI] [PubMed] [Google Scholar]
- 10.Floeter MK, Sholomenko GN, Gossard JP, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res. 1993;92:407–419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- 11.Gossard JP, Floeter MK, Degtyarenko AM, Simon ES, Burke RE. Disynaptic vestibulospinal and reticulospinal excitation in cat lumbosacral motoneurons: modulation during fictive locomotion. Exp Brain Res. 1996;109:277–288. doi: 10.1007/BF00231787. [DOI] [PubMed] [Google Scholar]
- 12.Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res. 1968;10:477–480. doi: 10.1016/0006-8993(68)90221-7. [DOI] [PubMed] [Google Scholar]
- 13.Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from the vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Exp Brain Res. 1971;12:457–479. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- 14.Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallen P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 15.Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habaguchi T, Takakusaki K, Saitoh K, Sugimoto J, Sakamoto T. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: II. Functional organization within the medullary reticular formation with respect to postsynaptic inhibition of forelimb and hindlimb motoneurons. Neuroscience. 2002;113:65–77. doi: 10.1016/s0306-4522(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 17.Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol (Lond) 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holstege G, Kuypers HG, Boer RC. Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res. 1979;171:329–333. doi: 10.1016/0006-8993(79)90337-8. [DOI] [PubMed] [Google Scholar]
- 19.Hoover JE, Durkovic RG. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens Mot Res. 1992;9:211–226. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, McCarley RW. Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res. 1987;409:97–110. doi: 10.1016/0006-8993(87)90745-1. [DOI] [PubMed] [Google Scholar]
- 21.Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- 22.Jankowska E, Roberts W. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol (Lond) 1972;222:623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kausz M. Arrangement of neurons in the medullary reticular formation and raphe nuclei projecting to thoracic, lumbar and sacral segments of the spinal cord in the cat. Anat Embryol (Berl) 1991;183:151–163. doi: 10.1007/BF00174396. [DOI] [PubMed] [Google Scholar]
- 24.Lund S, Pompeiano O. Descending pathways with monosynaptic action on motoneurones. Experientia. 1965;21:602–603. doi: 10.1007/BF02151558. [DOI] [PubMed] [Google Scholar]
- 25.Lund S, Pompeiano O. Monosynaptic excitation of alpha motoneurones from supraspinal structures in the cat. Acta Physiol Scand. 1968;73:1–21. doi: 10.1111/j.1748-1716.1968.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama K, Mori S. Lumbar interneurons involved in the generation of fictive locomotion in cats. Ann NY Acad Sci. 1998;860:441–443. doi: 10.1111/j.1749-6632.1998.tb09070.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama K, Ohta Y, Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1988;460:124–141. doi: 10.1016/0006-8993(88)91212-7. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, Kimura H. Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1993;17:9–21. doi: 10.1016/0168-0102(93)90024-k. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol. 1997;377:234–250. [PubMed] [Google Scholar]
- 30.Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- 31.McCarley RW, Ito K, Rodrigo-Angulo ML. Physiological studies of brainstem reticular connectivity. II. Responses of mPRF neurons to stimulation of mesencephalic and contralateral pontine reticular formation. Brain Res. 1987;409:111–127. doi: 10.1016/0006-8993(87)90746-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitani A, Ito K, Mitani Y, McCarley RW. Morphological and electrophysiological identification of gigantocellular tegmental field neurons with descending projections in the cat: I. Pons. J Comp Neurol. 1988a;268:527–545. doi: 10.1002/cne.902680405. [DOI] [PubMed] [Google Scholar]
- 33.Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol. 1988b;268:546–566. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- 34.Mitani A, Ito K, Mitani Y, McCarley RW. Morphological and electrophysiological identification of gigantocellular tegmental field neurons with descending projections in the cat: II. Bulb. J Comp Neurol. 1988c;274:371–386. doi: 10.1002/cne.902740307. [DOI] [PubMed] [Google Scholar]
- 35.Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Cereballar-induced locomotion: reticulospinal control of spinal rhythm generating mechanisms in cats. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal mechanisms for generating locomotor activity. New York Academy of Sciences; New York: 1998. pp. 94–106. [DOI] [PubMed] [Google Scholar]
- 36.Munson JB, Fleshman JW, Sypert GW. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980;44:713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- 37.Nyberg-Hansen R. Sites and mode of termination of reticulo-spinal fibers in the cat. J Comp Neurol. 1965;124:71–99. doi: 10.1002/cne.901240107. [DOI] [PubMed] [Google Scholar]
- 38.Orlovsky GN. Connexion of the reticulo-spinal neurons with the “locomotor sections” of the brain stem. Biofizika. 1970;15:171–177. [PubMed] [Google Scholar]
- 39.Perreault MC, Enriquez-Denton M, Hultborn H. Proprioceptive control of extensor activity during fictive scratching and weight support compared to fictive locomotion. J Neurosci. 1999;19:10966–10976. doi: 10.1523/JNEUROSCI.19-24-10966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson BW, Pitts NG, Fukushima K, Mackel R. Reticulospinal excitation and inhibition of neck motoneurons. Exp Brain Res. 1978;32:471–489. doi: 10.1007/BF00239548. [DOI] [PubMed] [Google Scholar]
- 41.Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- 42.Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100:297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- 43.Scheibel ME, Scheibel AB. Spinal motorneurons, interneurons and Renshaw cells. A Golgi study. Arch Ital Biol. 1966;104:328–353. [Google Scholar]
- 44.Shapovalov AI. Posttetanic potentiation of monosynaptic and disynaptic actions from supraspinal structures on lumbar motoneurons. J Neurophysiol. 1969;32:948–959. doi: 10.1152/jn.1969.32.6.948. [DOI] [PubMed] [Google Scholar]
- 45.Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 46.Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol. 1969;32:743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]