Abstract
In this review, the authors discuss some recent findings that bear on the issue of recovery of function after corticospinal tract lesions. Conventionally the corticospinal tract is considered to be a crossed pathway, in keeping with the clinical findings that damage to one hemisphere, for example, in stroke, leads to a contralateral paresis and, if the lesion is large, a paralysis. However, there has been great interest in the possibility of compensatory recovery of function using the undamaged hemisphere. There are several substrates for this including ipsilaterally descending corticospinal fibers and bilaterally operating neuronal networks. Recent studies provide important evidence bearing on both of these issues. In particular, they reveal networks of neurons interconnecting two sides of the gray matter at both brainstem and spinal levels, as well as intrahemispheric transcallosal connections. These may form “detour circuits” for recovery of function, and here the authors will consider some possibilities for exploiting these networks for motor control, even though their analysis is still at an early stage.
Keywords: Pyramidal tract, Spinal cord, Interneurons, Reticular formation, Motor system
Interest in the contributions of the motor cortex to ipsilateral voluntary movements stems from both clinical and basic studies. It is well known that injury to the motor cortex of one hemisphere or the corticospinal tract arising from it results in impairments of movements on the other side of the body, ranging from a weak and transient deterioration of precision and strength to a practically complete and lasting paralysis, depending on the species and the extent of the injury. However, there is an increasing body of evidence that the impairments are not only contralateral but also ipsilateral (see, for instance, Yelnik and others 1996; Marque and others 1997; Kim and others 2003; Yarosh and others 2004). This might indicate a contribution of corticospinal neurons to ipsilateral movements, which suggests a potential involvement of neurons in the intact hemisphere in the recovery of motor functions after injuries to the contralaterally projecting corticospinal neurons. However, there is no general consensus with respect to this possibility (for recent reviews, see, e.g., Hallett 2001; Chen and others 2002; Serrien and others 2004; Cauraugh and Summers 2005). Many studies have used fMRI to image blood flow changes in the undamaged hemisphere after stroke, as an indication of its involvement when attempts to move the ipsilateral limbs are made. Enhanced activity has been reported in ipsilateral sensorimotor, premotor, and/or supplementary motor areas but was not well correlated with recovery. This activity could relate to many aspects of altered function, for example, altered transcallosal interactions or altered postural commands, or it could be a response to a nonfunctioning system, as well as relate to the enhanced use of a system that can mediate descending commands for ipsilateral movement. Furthermore, in some cases, the enhancement of ipsilateral activity was strongest in the initial stages of recovery, or even negatively related to its progress (see, e.g., Feydy and others 2002; Foltys and others 2003; Serrien and others 2004). Similarly, transcranial magnetic stimulation has been reported to evoke responses in ipsilateral muscles after stroke, but these were not always seen and were more often seen in patients with poor than with good recovery (see, e.g., Turton and others 1996; Netz and others 1997; Caramia and others 2000).
Basic anatomical and physiological studies of corticospinal neurons have traditionally focused on their contralateral actions. A major reason for this was the predominance of contralateral corticospinal projections seen in classic neuroanatomical studies. Furthermore, only the crossed axons were found to form direct synaptic contacts with primate limb motoneurons (for references, see Porter and Lemon 1993), and direct, monosynaptically evoked excitation of motoneurons has long attracted more attention than the more difficult-to-analyze polysynaptic actions of corticospinal neurons. The results of the most influential studies of Woolsey’s group, that stimulation of the motor cortex evokes movements of contralateral but not of ipsilateral extremities (e.g., Woolsey and others 1979), added to the predominant interest in the crossed actions of corticospinal neurons. However, the early failures to evoke movements of ipsilateral extremities might have been related to the use of barbiturate-anesthetized animals. This is suggested by the fact that ipsilateral movements have been evoked in the awake man, although primarily in proximal muscles (Wassermann and others 1991; Turton and others 1996; Bawa and others 2004). The possibility of evoking ipsilateral movements in awake animals has apparently not been reported, usually only contralateral EMG being sampled while analyzing the effects of microstimulation in the primary motor cortex in awake cats and primates (see Porter and Lemon 1993).
Despite this focus on the contralateral actions of corticospinal neurons, there is growing evidence for the joint use of both hemispheres in the control of one limb, through presently ill-defined pathways (see Cauraugh and Summers 2005). Recent studies have also revealed several brainstem and spinal substrates for potential ipsilateral actions of corticospinal neurons.
Ipsilateral Corticospinal Projections and Their Plasticity
It has been repeatedly demonstrated since the pioneering neuroanatomical studies of Nyberg-Hansen (1966) and Kuypers and Brinkman (1970) that although the large majority of pyramidal tract (PT) neurons project to the spinal cord contralaterally, ipsilateral projections also exist. They have been found to extend throughout the spinal cord from cervical to lumbar segments (Ralston and Ralston 1985; Dum and Strick 1996; Armand and others 1997; Lacroix and others 2004). In many species, including primates, cats, and rats, corticospinal axons descend in both the ipsilateral lateral funiculus and the ipsilateral ventral funiculus. Humans are particularly interesting in that as many as 30% of the corticospinal axons may descend as the ipsilateral ventral corticospinal tract in some individuals (Nathan and others 1990).
Although ipsilateral trajectories of corticospinal fibers are well established, there is less information on the terminations of these fibers. Inasmuch as some corticospinal fibers (descending on either side of the cord) cross at a spinal level, both ipsilaterally and contralaterally descending fibers may terminate in the ipsilateral gray matter.
In primates, the ipsilateral corticospinal descending fibers originate from both the primary motor cortex and the other cortical areas (Kuypers and Brinkman 1970; Armand and Kuypers 1980; Armand and others 1985; Ralston and Ralston 1985; Dum and Strick 1991; Galea and Darian-Smith 1994, 1997; Dum and Strick 1996; Armand and others 1997; Lacroix and others 2004). Terminal projection areas of neurons in the motor and supplementary motor areas (SMAs) are generally similar, as illustrated in Figure 1A* and B*, although the SMA projections are much weaker. The highest density of ipsilateral terminals of corticospinal tract fibers descending on either side of the spinal cord is in the region of lamina VIII and the medial part of lamina VII, both in the cervical (Fig. 1) and lumbar (Fig. 2) segments. A major cell group located in lamina VIII are commissural interneurons, which project across the cord. Ipsilateral corticospinal actions that target these interneurons would thus affect contralateral rather than ipsilateral movements. Ipsilateral corticospinal terminals located outside lamina VIII have been reported less frequently. Using the sensitive anterograde tracer biotinylated dextran amine (Lacroix and others 2004) has described such terminals in laminae V to VII and IX, in addition to lamina VIII in the lumbar segments of the macaque monkey. In laminae V to VII, the terminals would be likely to contact ipsilaterally operating premotor interneurons that are numerous at these locations (see Jankowska 1992). In lamina IX, the sparse but clearly labeled terminals of ipsilateral fibers were found, and some were on large neurons in the motor nuclei (in keeping with data of Kucera and Wiesendanger 1985). If these large neurons are motoneurons, then these connections might be used for the direct control of ipsilateral motoneurons. However, the number of ipsilateral terminals is small; they constitute in total only about 10% of the contralateral ones.
Fig. 1.
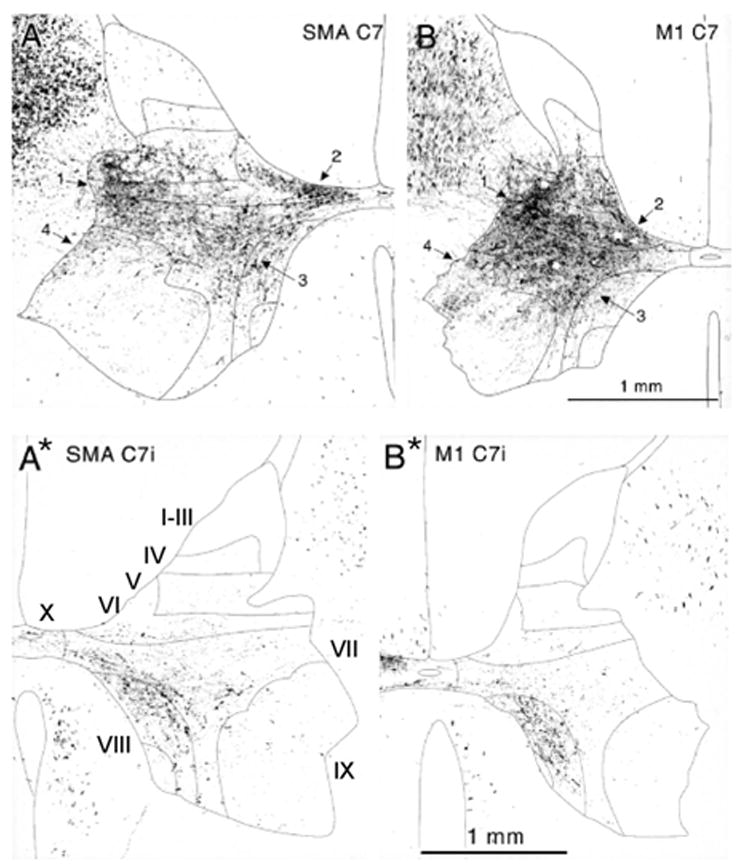
Comparison of contralateral (A, B) and ipsilateral (A*, B*) terminations of corticospinal neurons from the primary motor (M1) and supplementary motor (SMA) cortical areas in the C7 segment of the spinal cord in the macaque monkeys. Inverted photomicrographs taken under dark-field/polarized light of TMD (tetramethylbenzidine) labeling after WGA-HRP (wheat germ agglutinin-horseradish peroxidise) labeling after injections into M1 and SMA by Dum and Strick (1996). Numbered arrows indicate four densest terminal projection regions. Reproduced with permission of The Society for Neuroscience (copyright 1996). The thin lines indicate borders between Rexed’s laminae (Rexed 1954) as indicated in A*.
Fig. 2.
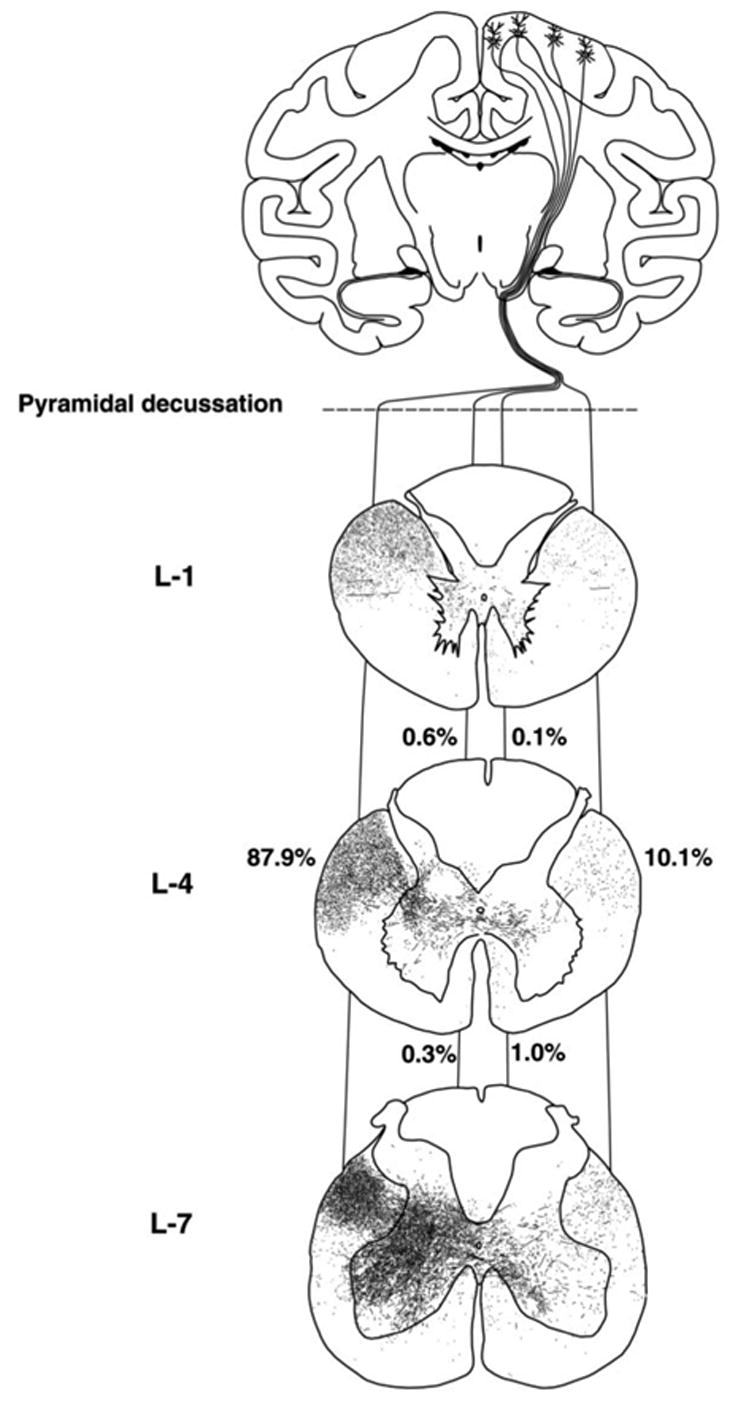
Comparison of contralateral and ipsilateral corticospinal projections and terminals at the L1 to L7 segmental levels of the spinal cord in a macaque monkey. Labeling after an injection of the anterograde tracer BDA (biotinylated dextranamine) into the right primary motor cortex by Lacroix and others (2004). Reproduced from Figure 10 in Lacroix and others (2004) with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Ipsilateral corticospinal projections have also been found in the cat, with similar proportions distributed ipsilaterally and contralaterally as in the primate (about 1:10; Armand and Kuypers 1980; Theriault and Tatton 1989). Also as in primates, the principal ipsilateral terminal projection areas were lamina VIII and the medial part of lamina VII (Flindt-Egebak 1979; Armand and others 1985; Li and Martin 2000). However, in cats, the origin of ipsilaterally projecting corticospinal neurons was found to be somewhat more narrow than in primates, primarily in the intermediate but not in more medial or lateral parts of the motor cortex (area 4) (Armand and Kuypers 1980; Armand and others 1985). Only very sparse ipsilateral projections from other sensorimotor areas have been reported (for references, see Armand and Kuypers 1980; Porter and Lemon 1993).
Ipsilateral projections depend on several factors, a key issue being the developmental stage. In immature cats and rats (see Theriault and Tatton 1989; Alisky and others 1992; Li and Martin 2000), ipsilateral projections are widespread and individual fibers often terminate bilaterally. The disappearance of many ipsilateral axons as the system matures depends on competition for synaptic targets and has been exploited in an elegant series of studies in the cat (Martin and others 1999; Friel and Martin 2005) and reviewed recently (Martin 2005). It is less clear whether similar competition occurs in primates. The very few cases in which infant nonhuman primates have been investigated are individual snapshots but have not revealed bilateral terminations (Galea and Darian-Smith 1995; Armand and others 1997). There are, however, suggestions that bilateral projections appear early in man (see Eyre and others 2001). A key feature is that after a developmental period in which competition occurs, most ipsilateral projections are eliminated.
Any factors that contribute to the withdrawal or to the increase in the density of ipsilateral projections may be of particular interest for the potential replacement of the contralateral actions of corticospinal neurons lost after injury by ipsilateral actions. As repeatedly pointed out, ipsilaterally distributed terminal branches of corticospinal tract neurons are a readily available source from which sprouting could enhance actions of these neurons during recovery following injuries of the contralaterally projecting neurons (Dum and Strick 1996; Galea and Darian-Smith 1997; Weidner and others 2001; Lacroix and others 2004). After the initial developmental period in which ipsilateral projections are competitively removed, there is much less plasticity and less opportunity for sprouting. In humans, as well as in animals, motor cortex damage early in development can accordingly often be alleviated by ipsilateral corticospinal projections, but the ipsilateral takeover only follows very early lesions (e.g., Benecke and others 1991; Carr and others 1993; Maegaki and others 1997; Nezu and others 1999; Eyre and others 2001; Thickbroom and others 2001; Staudt and others 2002 Staudt and others 2004).
Ipsilateral Corticospinal Actions Relayed via Subcortical and Spinal Neurons
In addition to the direct targets of corticospinal fibers described above, the motor cortex can also influence ipsilateral motoneurons through relay neurons located in the brainstem and spinal cord. These possibilities are shown in the diagrams of Figures 3 to 6. For the sake of simplicity, ipsilateral target cells of the PT neurons are indicated in these diagrams on the left side, the right side being referred to as contralateral. The diagrams are less complicated than they look, as will hopefully appear at the end of this review.
Fig. 3.
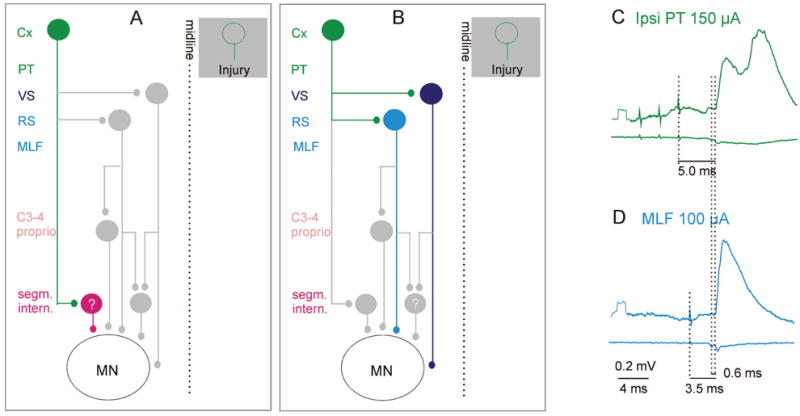
Relays between ipsilateral cortical neurons and motoneurons. A, Potential coupling between the motor cortex and spinal motoneurons via ipsilaterally located spinal premotor interneurons. The green cells represent ipsilaterally projecting cortical neurons that may substitute for injured corticospinal neurons on the opposite side (shown in the shaded box). The red cell in A represents premotor segmental interneurons contacted by the ipsilateral corticospinal neurons. B, Potential coupling between the motor cortex and spinal motoneurons via brainstem descending tract neurons. Light and dark blue cells in B represent reticulospinal and vestibulospinal neurons, respectively. In addition to direct connections with motoneurons, these also connect with spinal relay neurons, shown in gray, which are highlighted in Figures 4 to 6. C and D show averaged intracellular records from a hindlimb motoneuron (upper traces) and the surface of the spinal cord (lower traces) after administration of a K+ channel blocker 4-aminopyridine (4-AP, 0.2 mg/kg), which enhances synaptic transmission (see text). The green excitatory postsynaptic potentials (EPSPs) were evoked by stimulation of the ipsilateral pyramid (PT); blue, EPSP evoked by direct stimulation of reticulospinal fibers (MLF). Dotted lines indicate the stimulus artifacts, the descending volleys, and the onset of the EPSPs. The small additional latency of the green EPSP is consistent with a single additional synaptic relay. In this experiment, the corticospinal tract fibers were cut bilaterally at the C2 level, excluding the pathway proposed in A. These EPSPs should be evoked by activation of brain stem neurons via the connections shown in B. (E Jankowska, A Cabaj, and L-G Petterson, unpublished records). Cx = cortex; C3–4 proprio = propriospinal neurons in the third and fourth cervical segments; ipsi = ipsilateral; MLF = medial longitudinal fascicle; PT = pyramidal tract; RS = reticulospinal neurons; VS = vestibulospinal neurons.
Fig. 6.
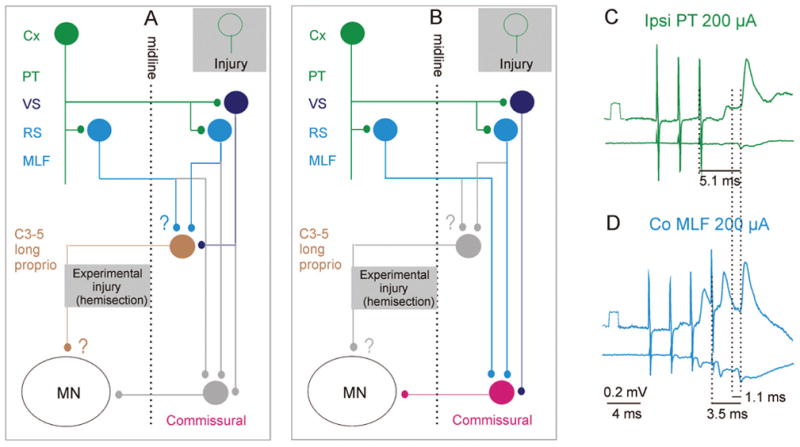
Diagram of relays in trisynaptic pathways between cortical neurons and ipsilateral motoneurons via contralateral descending tract neurons and spinal commissural neurons. A, Coupling via contralaterally projecting long propriospinal (C3–C5) neurons. B, Coupling via lumbar commissural interneurons. As in Figures 3 and 5, the green cells represent ipsilaterally projecting corticospinal neurons, acting via bilateral projections within the medulla. Light and dark blue cells represent reticulospinal and vestibulospinal neurons, respectively. The dark brown cell represents long propriospinal neurons in the third to fifth cervical segments, which are normally co-excited by contralateral corticospinal neurons and either ipsilaterally or both ipsilaterally and contralaterally located reticulospinal neurons (or by reticulospinal and vestibulospinal neurons). Details of input and output connections in these networks that have not yet been fully established are indicated by question marks. The red cell represents lumbar commissural interneurons acting on motoneurons on the opposite side. C and D, Intracellular records from a quadriceps motoneuron (upper traces) and from the surface of the spinal cord (lower traces), mediated by the red neurons. These recordings were made after a left-side hemisection at a low thoracic level (as shown in the gray box in B). Green, excitatory postsynaptic potentials (EPSPs) following the second and third stimuli applied to the ipsilateral pyramidal tract (PT). Blue, EPSPs evoked by medial longitudinal fascicle (MLF) stimuli. Temporal facilitation of EPSPs evoked by successive MLF stimuli and the segmental delay of 1.1 ms (from the first component of the MLF volley) indicates that the EPSPs were evoked via interneurons interposed between reticulospinal neurons and the motoneuron represented by the red cell in B. Modified from Jankowska and others (2005). Reproduced with permission of the Society for Neuroscience (copyright 2005). Other conventions as in Figures 3 and 5.
Actions Mediated via Ipsilaterally Located Segmental Interneurons
Very little information is available on the spinal target cells of the ipsilaterally projecting corticospinal neurons, other than where they are located (e.g., Figs. 1 and 2). However, premotor interneurons are numerous within the ipsilateral cortical projection areas, in laminae VI and VII as well as VIII, and are represented by the red cell in the diagram in Figure 3A. However, because the identity of these neurons is still undefined functionally, the cell is marked with the question mark.
Actions Mediated via Ipsilaterally Descending Reticulospinal and Vestibulospinal Neurons
Much more is known about the actions of PT neurons mediated by the activation of ipsilaterally descending reticulospinal and vestibulospinal neurons, represented by light and dark blue cells in Figure 3B, than of the potential spinal interneurons (Fig. 3A). The possibility that reticulospinal neurons are relay neurons for actions evoked from the motor cortex has been substantiated by both morphological and physiological studies. Direct collateral projections of corticospinal fibers to the nuclei from which reticulospinal tract fibers originate have been well demonstrated (Keizer and Kuypers 1984; Ugolini and Kuypers 1986; Keizer and Kuypers 1989; Matsuyama and Drew 1997; Kably and Drew 1998a). These connections were found from primary, premotor, and supplementary areas of the motor cortex, with more extensive projections from the areas controlling movements of proximal than of distal parts of the limbs (Keizer and Kuypers 1984; Kably and Drew 1998a). Neurons from both cerebral hemispheres were demonstrated to project to the medullary reticular formation and the caudal pontine nuclei but primarily from the ipsilateral hemisphere to the rostral pontine nuclei (Matsuyama and Drew 1997; Rho and others 1997).
Cortical stimulation evoked excitatory postsynaptic potentials (EPSPs) in a considerable proportion of reticulospinal neurons (Magni and Willis 1964; Peterson and others 1974; He and Wu 1985; Canedo and Lamas 1993). However, differences have been found in projections to the oral pontine and to more caudal reticular nuclei. In the medullary and caudal pontine nuclei, both monosynaptic and longer latency EPSPs were evoked from the ipsilateral and contralateral cortex. In contrast, in the oral pontine nuclei, monosynaptic and longer latency EPSPs were evoked from the ipsilateral cortex but only longer latency, possibly disynaptic EPSPs from the contralateral cortex (Peterson and others 1974). All these EPSPs were evoked by single stimuli, although action potentials usually required a train of three stimuli (Peterson and others 1974). Action potentials were evoked in more than one half of reticulospinal neurons in anesthetized animals and might thus contribute to movements in parallel with corticospinal actions.
The diagram in Figure 4E illustrates these connections with projections from the left PT to reticulospinal neurons on the left and right side (A and B, respectively) that descend on the right side. Projections from the right PT to both the same and other (C) reticulospinal neurons are also shown. Using the connections indicated in green and black, monosynaptic EPSPs evoked from one pyramid may summate with monosynaptic EPSPs evoked from the other pyramid and/or with disynaptic EPSPs evoked via recurrent action collaterals of other reticulospinal neurons and, in this way, increase the probability of activation of the reticulospinal neurons. EPSPs evoked by PT fibers may also increase the probability f synaptic activation of reticulospinal neurons via axon collaterals of other reticulospinal tract fibers stimulated within the medial longitudinal fascicle (MLF) under our experimental conditions, or activated by the various sources of input to these neurons under natural conditions (see Edgley and others 2004). This effect is illustrated by the facilitation of the descending reticulospinal volleys in Figure 4A to D (in the boxes) and is an indication of convergence of PT and reticulospinal neurons on reticulospinal neurons with axons in the MLF.
Fig. 4.
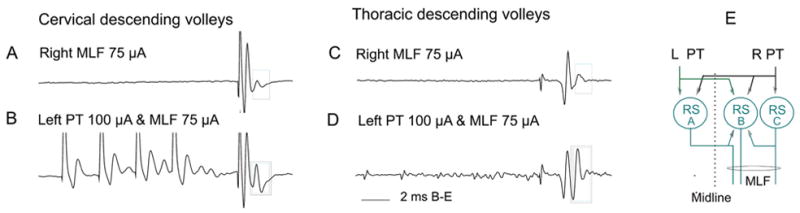
Facilitation of transmission between pyramidal tract (PT) fibers and reticulospinal neurons. Records of descending reticulospinal volleys from the C4 and Th 13 segments. A and C show descending volleys evoked by medial longitudinal fascicle (MLF) stimuli. The first component reflects action potentials evoked directly by stimulation of axons in MLF, whereas the second component is secondary to disynaptic activation of reticulospinal neurons, probably via axon collaterals of the MLF fibers. Note that only this component is facilitated by preceding PT stimulation. The diagram indicates connections between subgroups of reticulospinal (RS) neurons and between PT fibers and these neurons. Modified from Figure 3 in Edgley and others (2004). Reproduced with permission of The Society for Neuroscience (copyright 2004).
The next link, between reticulospinal neurons and spinal motoneurons, is also well established. Reticulospinal neurons have very strong actions upon neck motoneurons (Wilson and Yoshida 1969), and an involvement of reticulospinal neurons as relay neurons for disynaptic actions from the motor cortex was demonstrated in a two-step experiment: first, showing that the cortical actions remained unchanged after transection of the PTs in the caudal medulla, removing the spinal actions of the corticospinal fibers; and second, showing that they disappeared after a more rostral transection of the pyramid that disrupted corticospinal connections with medullary reticulospinal neurons (Alstermark and others 1985).
In the same way, monosynaptic excitation of limb motoneurons by reticulospinal neurons (Grillner and Lund 1968; Shapovalov 1969; Wilson and Yoshida 1969; Grillner and others 1971) would allow reticulospinal neurons to mediate disynaptic excitation of motoneurons by cortical neurons. However, monosynaptic EPSPs are only evoked from the reticulospinal tract in about 50% of flexor hindlimb motoneurons and are not particularly large (1–2 mV; Grillner and others 1971). The relative contribution of reticulospinal neurons to disynaptic cortical actions on these motoneurons may thus be moderate, but the observations of Gahery and Nieoullon (1978) that stimulation of the motor cortex evoked postural adjustment in the ipsilateral forelimb, together with movement of the contralateral forelimb, are most easily explained via a pathway of this type (see Massion 1992; Kably and Drew 1998b).
A potential use of vestibulospinal neurons as relay neurons in disynaptic pathways between cortical and spinal neurons is based on similar evidence for bilateral corticovestibular connections, although these are much stronger from premotor and parietal areas than from the primary motor cortex (for review, see Fukushima 1997) and for direct actions from vestibulospinal neurons to ipsilateral motoneurons (Grillner and Lund 1968; Shapovalov 1969; Wilson and Yoshida 1969; Grillner and others 1970). The actual contribution of vestibulospinal neurons to disynaptic corticospinal actions has not been investigated but is likely to be considerably smaller than that of the reticulospinal neurons because a relatively small number of neurons in motor and sensorimotor cortical areas project to the vestibular nuclei and a relatively small number of vestibular neurons are affected (Wilson and others 1999). Monosynaptic EPSPs evoked in motoneurons by vestibular stimulation were of similar amplitude to the reticulospinal EPSPs (1–2 mV) and were evoked in a similar proportion of motoneurons (although mainly extensors; Grillner and others 1971).
Actions Mediated via Ipsilaterally Descending Reticulospinal and/or Vestibulospinal Neurons and Spinal Relay Neurons
Brain stem relay neurons with ipsilaterally descending axons that are activated by cortical neurons will act not only directly on motoneurons but also indirectly via spinal interneurons, represented by the light brown and red cells in Figure 5A and B. These two cells represent propriospinal neurons and segmental interneurons, respectively.
Fig. 5.
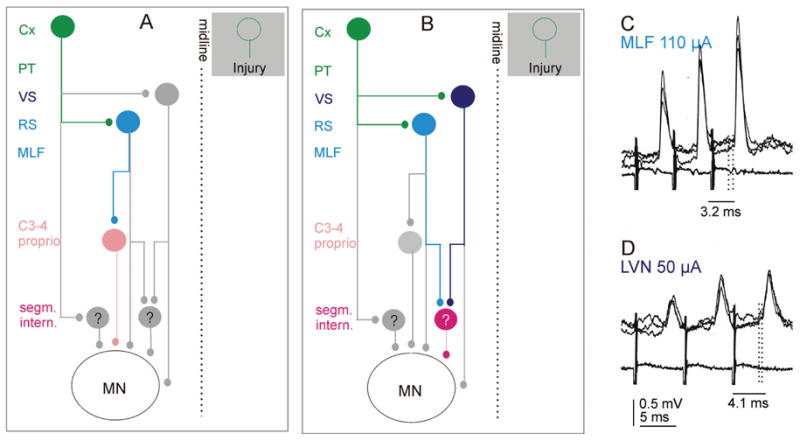
Diagram of relays in trisynaptic pathways between cortical neurons and ipsilateral motoneurons. A, trisynaptic coupling via ipsilaterally projecting reticulospinal and propriospinal neurons. B, trisynaptic coupling via ipsilaterally projecting reticulospinal and/or vestibulospinal neurons and segmental interneurons. As in Figure 3, the green cells represent ipsilaterally projecting corticospinal neurons, and the light and dark blue cells represent reticulospinal and vestibulospinal neurons. The brown cell represents propriospinal neurons in the third-fourth cervical segments, which are normally co-excited by contralateral corticospinal neurons and reticulospinal neurons. The red cell represents premotor segmental interneurons. They are known to exist but have not yet been identified (hence the question mark). Gray cells represent relay cells highlighted in other diagrams. C and D show intracellular recordings from an interneuron of the red type: monosynaptic excitatory postsynaptic potentials (EPSPs) evoked by stimulation of reticulospinal fibers (medial longitudinal fascicle [MLF]) and vestibulospinal fibers (LVN; Lateral Vestibular Nucleus). (HE Davies and SA Edgley, unpublished records.) Other conventions as in Figure 3C and D.
Propriospinal neurons located in the upper cervical (C3–C4) segments in the cat spinal cord have been investigated very extensively (for reviews, see Lundberg 1979; Alstermark and Lundberg 1992; Alstermark and Isa 2002). They are particularly important for the visuo-motor control of proximal forelimb muscles in object-directed movements, but some recent work also suggests that they are important in the control of primate hand movements (Sasaki and others 2004) and in recovery of motor functions in hemiparetic patients (Mazevet and others 2003; Stinear and Byblow 2004). These C3 to C4 propriospinal neurons project to forelimb motoneurons and to interneurons in laminae VI to VIII (Alstermark, Kummel, and others 1987). They have monosynaptic input from contralateral PT fibers (not shown in the figure), but ipsilateral PT fibers may act on them (disynaptically) via reticulospinal or rubrospinal tract neurons because individual neurons are co-excited by reticulospinal and rubrospinal fibers (Illert and others 1978; Illert and others 1981). Wide sources of input have also been found in C3 to C5 propriospinal neurons that project to the lumbar segments (Alstermark, Lundberg, and others 1987a, 1987b), but nothing is as yet known on their target cells.
Any interneurons that mediate disynaptic reticulospinal actions on motoneurons (see, e.g., Shapovalov and Gurevitch 1970; Grillner and others 1971; Floeter and others 1993) might also mediate trisynaptic corticospinal actions. However, very little is known about excitatory interneurons involved in these actions (see, e.g., Davies and Edgley 1994), more information being available about interneurons that mediate disynaptic inhibition, which include interneurons in inhibitory pathways between group Ib or group Ia afferents and motoneurons, respectively (Hultborn and others 1976; Takakusaki and others 1989; Takakusaki and others 2001). Similar arguments might apply to vestibulospinal actions, although likelihood of activation of vestibulospinal fibers from the motor cortex is low, as discussed above.
Actions Mediated via Contralaterally Descending Reticulospinal and/or Vestibulospinal Neurons and Spinal Relay Neurons
A relatively small number of reticulospinal tract fibers appear to give off axon collaterals that cross the midline in the spinal cord, and might contact both motoneurons and interneurons (Nyberg-Hansen 1965; Holstege and others 1979; Matsuyama and others 1999). The direct actions of these crossed axon collaterals on motoneurons are in addition weak and are evoked in only about 10% of motoneurons (Jankowska and others 2003); actions on interneurons have not been reported. The main actions of reticulospinal neurons on contralateral motoneurons should thus be evoked via commissural interneurons, which are located on the same side as the reticulospinal neurons and have axons that cross to the opposite side. Some of these interneurons project within only a few segments, whereas other ones belong to long propriospinal neurons. They are represented by the red and dark brown cells in Figure 6A and B and provide the substrate for the double-crossed connections between the corticospinal neurons and motoneurons.
The long propriospinal commissural neurons are located in the C3 to C5 segments. Input is direct from contralateral PT fibers (monosynaptic, not shown in the figure) as well as indirect from ipsilateral PT fibers (disynaptic, via reticulospinal neurons that are the main source of monosynaptic input to them together with vestibulospinal neurons; Alstermark, Lundberg, and others 1987a, 1987b; Alstermark and others 1991). The axons of these neurons cross immediately and descend contralaterally (within the ventral funiculus) as far as the lumbar segments (Alstermark, Lundberg, and others 1987b). However, there is so far no information on their target neurons or on their actions within the lumbosacral enlargement. It is also unknown how they relate to the previously investigated long propriospinal neurons located between the C3 and Th 10 segments (Jankowska and others 1973; Jankowska and others 1974) that were found to evoke monosynaptic EPSPs and disynaptic EPSPs or inhibitory postsynaptic potentials (IPSPs) in hindlimb motoneurons.
To investigate the possibility that commissural inter-neurons operating within the lumbosacral enlargement (represented by the red cell in Fig. 6) act as spinal relays for corticospinal neurons, we analyzed the synaptic actions evoked by stimulation of the ipsilateral PT on hindlimb motoneurons, after transecting the crossed corticospinal and any other ipsilaterally descending tract fibers at a low thoracic level (Edgley and others 2004; Jankowska and others 2005). Under our standard experimental conditions, these stimuli did not evoke synaptic actions in the motoneurons alone, but they did potently facilitate disynaptic PSPs evoked from the reticulospinal system via commissural interneurons (see Edgley and others 2004). Observations that provide evidence for trisynaptic corticospinal actions via this pathway are outlined in Figure 6B. First, records in Figure 7A to C show that even when no EPSPs followed PT stimulation (A), disynaptic EPSPs (B) evoked by stimulation of reticulospinal tract fibers in the contralateral MLF were potently facilitated by PT stimulation (C). The timing of this facilitation was appropriate for direct coupling between corticospinal and reticulospinal neurons (Edgley and others 2004). Effects of PT stimulation were also reflected in an increase in the amplitudes of descending volleys in indirectly activated reticulospinal neurons, as illustrated in Figure 4B and D. Stimulation of pyramids could by itself induce EPSPs in motoneurons when both pyramids were stimulated (Jankowska and others 2005). As shown in Figure 7D to F when stimulation of the ipsilateral PT was combined with stimulation of the contralateral PT, trisynaptic EPSPs appeared. Mutual facilitation of the actions from the two pyramids resulting in such EPSPs was found in the majority of motoneurons in which disynaptic EPSPs were evoked from MLF. The facilitation may thus be attributed to the spatial facilitation of actions from the two pyramids on common reticulospinal neurons. Finally, even separate stimulation of the ipsilateral PT became effective when synaptic transmission in the pathway between PT fibers and motoneurons was enhanced by the K+ channel blocker 4-aminopyridine (4-AP) (Jankowska and others 2005), as illustrated in Figure 7H. The similar latencies of the EPSPs evoked by PT and MLF stimulation with respect to the descending volleys (Fig. 6C and D) indicate that both are mediated by a single additional relay interneuron. The differences in latencies of EPSPs evoked by PT and MLF stimuli (1–1.6 ms with respect to the stimuli) are fully compatible with an additional conduction time along axon collaterals of PT fibers and a synaptic delay in synapses between them and reticulospinal neurons. (For details, see Edgley and others 2004; Jankowska and others 2005.)
Fig. 7.
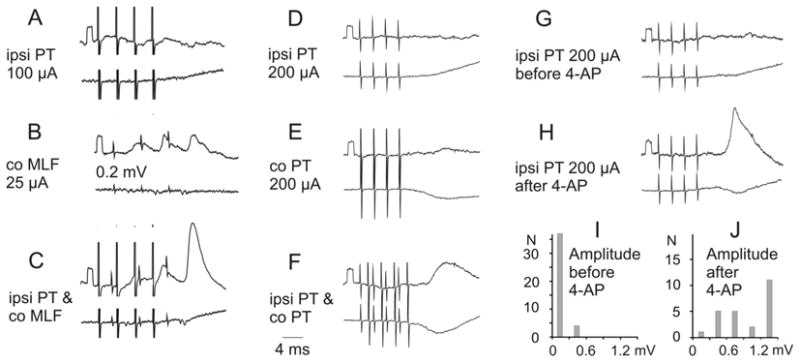
Trisynaptic excitation of motoneurons by ipsilateral (ipsi) pyramidal tract (PT) neurons. A–C, D–F, and G–H, Intracellular records from three motoneurons (upper traces) and records from the cord dorsum (lower traces). The responses were evoked via reticulospinal fibers and the segmental commissural neurons in the pathway indicated in Figure 6B. A–C, Facilitation of an excitatory postsynaptic potential (EPSP) evoked from medial longitudinal fascicle (MLF) by a preceding PT stimulation. D–F, Appearance of an EPSP following stimulation of both ipsilateral and contralateral PT. G and H, Appearance of an EPSP from the ipsilateral PT after application of 4-aminopyridine (4-AP) (0.3 mg/kg). Modified from Figure 6 in Edgley and others (2004) and from Figures 1 and 2 in Jankowska and others (2005). Reproduced with permission of the Society for Neuroscience (copyright 2004 and 2005).
The conclusion that there is a disynaptic coupling between reticulospinal neurons and contralateral motoneurons based on electrophysiological analysis has been substantiated by demonstration of axonal projections of individual commissural interneurons that were monosynaptically activated by reticulospinal tract fibers to large neurons in the contralateral motor nuclei (Bannatyne and others 2003). Immunocytohistochemical analysis of the transmitter content in terminals of commissural interneurons (Bannatyne and others 2003) revealed that this population includes both excitatory glutamatergic interneurons (Fig. 8D–F) and inhibitory glycinergic neurons (Fig. 8G–I).
Fig. 8.
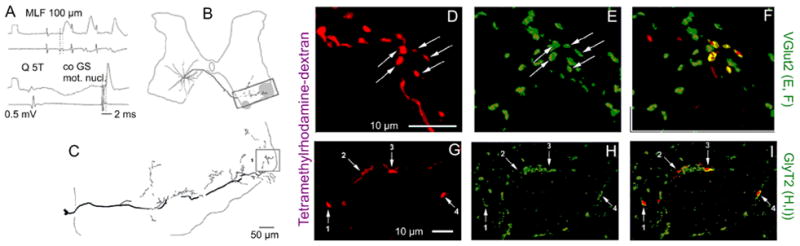
Examples of reconstructions of the axonal projections of commissural interneurons with monosynaptic input from medial longitudinal fascicle (MLF) and immunoreactivity of their terminals for glutamate and glycine transporters (VGLUT2 and GlyT2). A, Records from a commissural interneuron labeled with a mixture of tetramethylrhodamine and neurobiotin showing monosynaptic excitatory postsynaptic potentials (EPSPs) evoked by MLF stimulation, an inhibitory postsynaptic potential evoked by group II afferent stimulation (quadriceps nerve), and antidromic action potential evoked from the contralateral gastrocnemius-soleus motor nucleus. B and C, Location of the cell body of this interneuron, the trajectory of its axon, and the reconstruction of the most ventral axon collateral (on a larger scale). The contralateral motor nuclei are shaded. D–F, Single optical section confocal images of terminals showing rhodamine fluorescence (D) and immunoreactivity for VGLUT2 (E) and a merged view of D and E (F). G–H, A similar series of confocal images of GlyT2 immunoreactive axon terminals from a different neuron. Modified from Figures 4, 6, and 9 in Bannatyne and others (2003). Reproduced with permission of Blackwell Publishing.
Inhibition Associated With Excitation Evoked by Ipsilateral Corticospinal Actions
Until now, we have discussed only excitatory ipsilateral actions of corticospinal neurons. However, these were by no means the only actions these pathways could generate, and inhibition often accompanies excitation evoked by stimulation of pyramids. This was in particular the case of synaptic actions mediated by commissural interneurons (red cells in Fig. 6B). As shown previously, both excitatory and inhibitory commissural interneurons exist. Those mediating disynaptic inhibition of motoneurons of reticular origin could thus mediate trisynaptic inhibition evoked by corticospinal neurons, provided that these neurons activated reticulospinal neurons having inhibitory actions on motoneurons. Records in Figure 9 show that this is indeed the case.
Fig. 9.
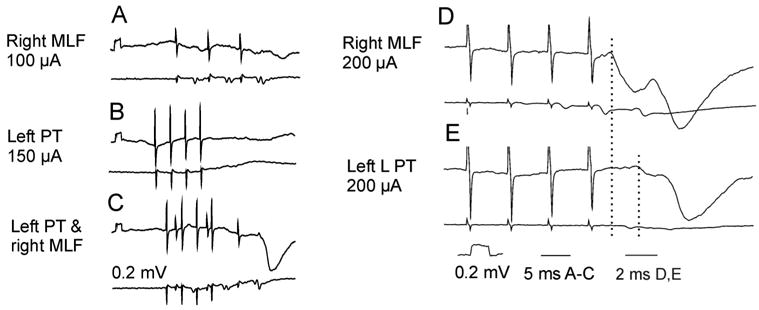
Examples of inhibitory ipsilateral corticospinal actions mediated by lumbar commissural interneurons. A–C and D–E, Records from two different sartorius motoneurons on the left side (upper traces) and from the cord dorsum (lower traces). A–C illustrate mutual facilitation of synaptic actions following stimulation of the right medial longitudinal fascicle (MLF) and of the ipsilateral (left) pyramidal tract (PT). D–E, Records allowing a comparison of the timing of inhibitory postsynaptic potentials (IPSPs) evoked by MLF and PT stimuli when their effectiveness was increased by 4-aminopyridine (4-AP). The latency of the MLF IPSP in both C and D was compatible with disynaptic coupling and that of the PT IPSP in E was 1.8 ms longer (compatible with one or two additional synaptic delays). All descending fibers on the left side of the spinal cord were transected at mid-thoracic level. Modified from Figure 10 in Edgley and others (2004) and from Figure 3 in Jankowska and others (2005). Reproduced with permission of the Society for Neuroscience (copyright 2004 and 2005).
Evidence That Reticulospinal Neurons Are Important for Relaying Ipsilateral Corticospinal Actions
The studies reviewed above have singled out the reticulospinal pathway as being of particular importance for the ipsilateral actions of corticospinal neurons. Comparison with other systems is difficult because there have been no similar studies of the potential contributions of other neuronal systems. However, there are reports of considerable differences between the effects of injuries of corticospinal axon neurons at a level rostral to where they give off collaterals in the brainstem and of more caudal injuries. After a complete but selective lesion of the PTs in the medulla, a considerable degree of recovery occurs, both in primates (Lawrence and Kuypers 1968) and in other species (Gorska and Sybirska 1980; Alstermark and others 1989; Whishaw and others 1998). The recovery involves both proximal and distal muscles, allowing, for example, whole-hand grasping movements. These deficits were much weaker and the recovery was faster after lesions of corticospinal fibers caudally in the medulla and even weaker after lesions within the C2 segment (Alstermark and others 1981; Sasaki and others 2004). These results indicate that the actions of corticospinal neurons within the spinal cord may, to a great extent, be substituted by actions of more rostrally located brainstem and upper spinal propriospinal neurons (brown neurons in Fig. 5A). Because input to these neurons is from reticulospinal but not vestibulospinal neurons, this is another argument in favor of the importance of reticulospinal neurons.
Although the reticulospinal pathways are one of the oldest descending pathways in phylogenetic terms, their role remains incompletely understood. Microstimulation of the reticular formation frequently evoked movements of more than one limb, or of a limb and the head, further suggesting a role in integrated whole body movement (see Drew and Rossignol 1990; Drew 1991; Mori and others 1992); these movements were mainly in the proximal joints and involved several muscles. Because motor cortex projections to the reticular formation are in addition very extensive (see, e.g., Magni and Willis 1964; Keizer and Kuypers 1984, 1989; Kably and Drew 1998a), projections through this system might not be appropriate for producing individuated, fractionated movements of distal parts of the limbs but might be involved in more general motor patterns. Perhaps because of these effects, corticoreticular projections are usually discussed in terms of a role in generating the coordinated postural adjustments that accompany movements driven by the motor cortex (see Luccarini and others 1990; Massion 1992). However, as pointed out by Schepens and Drew (2003), there are both reticulospinal neurons that have activity with a close temporal correlation with movement and others with a close temporal correlation with postural adjustment that accompanies the movement, suggesting that the reticulospinal system has a fundamental role in integrating commands for both movement and its postural requirements. Ipsilateral corticospinal actions mediated by reticulospinal neurons may thus take advantage of these integrative functions.
Can Ipsilateral Actions of Corticospinal Neurons Be Enhanced?
Inasmuch as the ipsilateral actions of intact corticospinal neurons are much weaker under normal circumstances than the crossed actions, their involvement in the recovery of motor functions that are deficient after injuries of contralaterally projecting corticospinal neurons might require that they be strengthened. The evidence that recovery of function can be very good if function in one hemisphere is compromised early in development, in both man and experimental animals, should be tempered by the observations that this major remodeling only occurs for lesions that occur very early in development, where corticospinal plasticity is much greater than it is in more mature animals. Any recovery in more mature animals will be restricted to local sprouting, hence the importance of alternative networks of connections that exist in the adult. Actions of ipsilateral corticospinal neurons might be enhanced at different sites secondary to sprouting from terminal branches of corticospinal neurons and by facilitation of activation of their relay neurons. In this closing section, we would like to point out some more general means for enhancing the activity in these pathways. There is already evidence for spinal “detour circuits,” or recurrent positive feedback circuits, that would allow plastic changes in spinal interneuronal circuits and their input connections (Galea and Darian-Smith 1997; Bareyre and others 2004). A route from motor cortex through reticulospinal tract neurons and commissural neurons to ipsilateral motoneurons may be a particularly convenient target “detour” pathway for rehabilitation interventions. Whether these could involve both distal and proximal or only proximal movements is an open question.
Previous studies have shown that the activation of the commissural interneurons involved is powerfully modulated by presynaptic inhibition (Edgley and others 2003) and by monoamine systems (Hammar and others 2004). Both of these might provide a potential means for influencing the general excitability of these networks, for example, pharmacologically. Modulation by serotonin and noradrenaline is a particularly interesting possibility, in that both enhance the activation of commissural interneurons by reticulospinal fibers (Hammar and others 2004). However, serotonin and noradrenaline also act on other spinal interneurons and motoneurons, and both these actions and their effects at supraspinal levels (e.g., on the reticulospinal neurons themselves) have to be considered. The recently reported potentiation of actions of PT neurons on ipsilateral motoneurons by a K+ channel blocker 4-AP (Jankowska and others 2005) illustrated in Figure 8G and H is also promising in this respect, in that 4-AP has already proved a beneficial treatment in other cases of motor deficits: in patients with multiple sclerosis and after various spinal cord injuries (for review, see Nashmi and Fehlings 2001). In addition, effects of 4-AP were reported to outlast its elimination time (Hansebout and others 1993). This opens possibilities for plastic changes in the operation of the involved neuronal networks to assist the recovery. The use of different rehabilitation procedures to raise and maintain a high level of activity in pathways involving these networks would assist any of these procedures. However, our knowledge of how this could be achieved will depend on further analysis of the operation of these networks and their plasticity.
Footnotes
The authors’ studies were financed by the National Institutes of Health (USA), the Swedish Research Council, and the Biotechnology and Biological Sciences Research Council (UK).
References
- Alisky JM, Swink TD, Tolbert DL. The postnatal spatial and temporal development of corticospinal projections in cats. Exp Brain Res. 1992;88:265–76. doi: 10.1007/BF02259101. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T. Premotoneuronal and direct corticomotoneuronal control in the cat and macaque monkey. Adv Exp Med Biol. 2002;508:281–97. doi: 10.1007/978-1-4615-0713-0_34. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Lundberg A, Pettersson LG, Tantisira B. The effect of low pyramidal lesions on forelimb movements in the cat. Neurosci Res. 1989;7:71–5. doi: 10.1016/0168-0102(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Tantisira B. Pyramidal excitation in long propriospinal neurones in the cervical segments of the cat. Exp Brain Res. 1991;84:569–82. doi: 10.1007/BF00230969. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kummel H, Pinter MJ, Tantisira B. Branching and termination of C3–C4 propriospinal neurones in the cervical spinal cord of the cat. Neurosci Lett. 1987;74:291–6. doi: 10.1016/0304-3940(87)90312-0. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A. The C3–C4 propriospinal system: target-reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle afferents and spinal control of movement. Oxford, UK: Pergamon; 1992. pp. 327–54. [Google Scholar]
- Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42:299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Pinter M, Sasaki S. Subpopulations and functions of long C3–C5 propriospinal neurones. Brain Res. 1987a;404:395–400. doi: 10.1016/0006-8993(87)91402-8. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Pinter M, Sasaki S. Long C3–C5 propriospinal neurones in the cat. Brain Res. 1987b;404:382–8. doi: 10.1016/0006-8993(87)91400-4. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Pinter MJ, Sasaki S. Pyramidal effects in dorsal neck motoneurones of the cat. J Physiol. 1985;363:287–302. doi: 10.1113/jphysiol.1985.sp015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand J, Holstege G, Kuypers HG. Differential corticospinal projections in the cat. An autoradiographic tracing study. Brain Res. 1985;343:351–5. doi: 10.1016/0006-8993(85)90754-1. [DOI] [PubMed] [Google Scholar]
- Armand J, Kuypers HG. Cells of origin of crossed and uncrossed corticospinal fibers in the cat: a quantitative horseradish peroxidase study. Exp Brain Res. 1980;40:23–34. doi: 10.1007/BF00236659. [DOI] [PubMed] [Google Scholar]
- Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci. 1997;17:251–66. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural inter-neurons mediating crossed reticulospinal actions identified by immunocytochemistry. Eur J Neurosci. 2003;18:2273–84. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–77. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res. 2004;158:385–90. doi: 10.1007/s00221-004-2031-x. [DOI] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–26. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Canedo A, Lamas JA. Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol. 1993;463:475–89. doi: 10.1113/jphysiol.1993.sp019606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol. 2000;111:1990–6. doi: 10.1016/s1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(Pt 5):1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–20. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–73. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol (Lond) 1994;479:463–73. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Functional organization within the medullary reticular formation of the intact unanesthetized cat: III. Microstimulation during locomotion. J Neurophysiol. 1991;66:919–38. doi: 10.1152/jn.1991.66.3.919. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat: I. Movements evoked by microstimulation. J Neurophysiol. 1990;64:767–81. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–89. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–25. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–13. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from group II muscle afferents. J Physiol. 2003;552:961–74. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Flindt-Egebak P. The corticofugal projections from the sensorimotor cortex to the spinal cord. A neuroanatomical and autoradiographical study in the cat with some methodological comments. J Hirnforsch. 1979;20:363–73. [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard JP, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res. 1993;92:407–19. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, et al. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–15. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- Friel KM, Martin JH. Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol. 2005;485:43–56. doi: 10.1002/cne.20483. [DOI] [PubMed] [Google Scholar]
- Fukushima K. Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res. 1997;117:1–16. doi: 10.1007/pl00005786. [DOI] [PubMed] [Google Scholar]
- Gahery Y, Nieoullon A. Postural and kinetic coordination following cortical stimuli which induce flexion movements in the cat’s limbs. Brain Res. 1978;149:25–37. doi: 10.1016/0006-8993(78)90585-1. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–94. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5:518–40. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Corticospinal projection patterns following unilateral section of the cervical spinal cord in the newborn and juvenile macaque monkey. J Comp Neurol. 1997;381:282–306. [PubMed] [Google Scholar]
- Gorska T, Sybirska E. Effects of pyramidal lesions on forelimb movements in the cat. Acta Neurobiol Exp (Wars) 1980;40:843–59. [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. The vestibulospinal tract. Effects on alpha-motoneurones in the lumbosacral spinal cord in the cat. Exp Brain Res. 1970;10:94–120. doi: 10.1007/BF00340521. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from the vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Exp Brain Res. 1971;12:457–79. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. The origin of a descending pathway with monosynaptic action on flexor motoneurones. Acta Physiol Scand. 1968;74:274–84. doi: 10.1111/j.1748-1716.1968.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Hallett M. Functional reorganization after lesions of the human brain: studies with transcranial magnetic stimulation. Rev Neurol (Paris) 2001;157:822–6. [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–16. doi: 10.1111/j.l460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansebout RR, Blight AR, Fawcett S, Reddy K. 4-Aminopyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J Neurotrauma. 1993;10:1–18. doi: 10.1089/neu.1993.10.1. [DOI] [PubMed] [Google Scholar]
- He XW, Wu CP. Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res. 1985;61:109–16. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG, Boer RC. Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res. 1979;171:329–33. doi: 10.1016/0006-8993(79)90337-8. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones: III. Effects from supraspinal pathways. Acta Physiol Scand. 1976;96:368–91. doi: 10.1111/j.1748-1716.1976.tb10206.x. [DOI] [PubMed] [Google Scholar]
- Illert M, Jankowska E, Lundberg A, Odutola A. Integration in descending motor pathways controlling the forelimb in the cat: 7. Effects from the reticular formation on C3–C4 propriospinal neurones. Exp Brain Res. 1981;42:269–81. doi: 10.1007/BF00237494. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat: 5. Properties of and monosynaptic excitatory convergence on C3–C4 propriospinal neurones. Exp Brain Res. 1978;33:101–30. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from pro-prioceptors. Prog Neurobiol. 1992;38:335–78. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson L-G. How to enhance ipsilateral actions of pyramidal tract neurons? J Neurosci. 2005;25:7401–5. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–78. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Roberts WJ, Stuart D. A long propriospinal system with direct effect on motoneurones and on interneurones in the cat lumbosacral cord. Exp Brain Res. 1974;21:169–94. doi: 10.1007/BF00234388. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Stuart D. Propriospinal control of last order interneurones of spinal reflex pathways in the cat. Brain Res. 1973;53:227–31. doi: 10.1016/0006-8993(73)90786-5. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat: I. Projection patterns and collaterization. J Neurophysiol. 1998a;80:389–405. doi: 10.1152/jn.1998.80.1.389. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat: II. Discharge activity of neurons in area 4 during voluntary gait modifications. J Neurophysiol. 1998b;80:406–24. doi: 10.1152/jn.1998.80.1.406. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to lower brain stem reticular formation in cat. Exp Brain Res. 1984;54:107–20. doi: 10.1007/BF00235823. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Exp Brain Res. 1989;74:311–8. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch Phys Med Rehabil. 2003;84:719–24. doi: 10.1016/s0003-9993(02)04973-0. [DOI] [PubMed] [Google Scholar]
- Kucera P, Wiesendanger M. Do ipsilateral corticospinal fibers participate in the functional recovery following unilateral pyramidal lesions in monkeys? Brain Res. 1985;348:297–303. doi: 10.1016/0006-8993(85)90448-2. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in therhesus monkey. Brain Res. 1970;24:29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, et al. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–61. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey: I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of differential projections from the caudal and rostral motor cortex subregions. Exp Brain Res. 2000;134:187–98. doi: 10.1007/s002210000454. [DOI] [PubMed] [Google Scholar]
- Luccarini P, Gahery Y, Pompeiano O. Cholinoceptive pontine reticular structures modify the postural adjustments during the limb movements induced by cortical stimulation. Arch Ital Biol. 1990;128:19–45. [PubMed] [Google Scholar]
- Lundberg A. Integration in propriospinal motor centre controlling the forelimb in the cat. In: Asanuma H, Wilson VS, Asanuma H, Wilson VS, editors. Integration in the nervous system. Tokyo: Igaru-Shoin; 1979. pp. 47–65. [Google Scholar]
- Maegaki Y, Maeoka Y, Seki A, Ueno M, Yamamoto T, Takeshita K. Facilitation of ipsilateral motor pathways during recovery from hemiplegia in two adolescent patients. Eur J Paediatr Neurol. 1997;1:79–84. doi: 10.1016/s1090-3798(97)80067-0. [DOI] [PubMed] [Google Scholar]
- Magni F, Willis WD. Cortical control of brain stem reticular neurons. Arch Ital Biol. 1964;102:418–33. [PubMed] [Google Scholar]
- Marque P, Felez A, Puel M, Demonet JF, Guiraud-Chaumeil B, Roques CF, et al. Impairment and recovery of left motor function in patients with right hemiplegia. J Neurol Neurosurg Psychiatry. 1997;62:77–81. doi: 10.1136/jnnp.62.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res. 1999;125:184–99. doi: 10.1007/s002210050673. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgarisleucoagglutinin. J Comp Neurol. 1997;389:617–41. doi: 10.1002/(sici)1096-9861(19971229)389:4<617::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–30. [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Kohyama J, Kobayashi Y, Takakusaki K. Neuronal constituents of postural and locomotor control systems and their interactions in cats. Brain Dev. 1992;14(Suppl):S109–20. [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–91. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC, Deacon P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain. 1990;113(Pt 2):303–24. doi: 10.1093/brain/113.2.303. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–86. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Takeshita S, Tanaka M. Functional recovery in hemiplegic cerebral palsy: ipsilateral electromyographic responses to focal transcranial magnetic stimulation. Brain Dev. 1999;21:162–5. doi: 10.1016/s0387-7604(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. Sites and mode of termination of reticulospinal fibers in the cat. J Comp Neurol. 1965;124:71–99. doi: 10.1002/cne.901240107. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. Functional organization of descending supra-spinal fibre systems to the spinal cord. Anatomical observations and physiological correlations. Ergeb Anat Entwicklungsgesch. 1966;39:3–48. [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M. Responses of pontomedullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res. 1974;21:19–44. doi: 10.1007/BF00234256. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford, UK: Clarendon; 1993. [Google Scholar]
- Ralston DD, Ralston HJ., 3rd The terminations of corticospinal tract axons in the macaque monkey. J Comp Neurol. 1985;242:325–37. doi: 10.1002/cne.902420303. [DOI] [PubMed] [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100:297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Cabana T, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary reticular formation of the cat: a quantitative retrograde tracing study. J Comp Neurol. 1997;388:228–49. doi: 10.1002/(sici)1096-9861(19971117)388:2<228::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–7. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90:3066–86. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol. 2004;190:425–32. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI. Posttetanic potentiation of monosynaptic and disynaptic actions from supraspinal structures on lumbar motoneurons. J Neurophysiol. 1969;32:948–59. doi: 10.1152/jn.1969.32.6.948. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI, Gurevitch NR. Monosynaptic and disynaptic reticulospinal actions on lumbar motoneurons of the rat. Brain Res. 1970;21:249–63. doi: 10.1016/0006-8993(70)90366-5. [DOI] [PubMed] [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–63. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–34. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Kohyama J, Matsuyama K, Mori S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience. 2001;103:511–27. doi: 10.1016/s0306-4522(00)00586-8. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Theriault E, Tatton WG. Postnatal redistribution of pericruciate motor cortical projections within the kitten spinal cord. Brain Res Dev Brain Res. 1989;45:219–37. doi: 10.1016/0165-3806(89)90041-2. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol. 2001;49:320–7. [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–28. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Kuypers HG. Collaterals of corticospinal and pyramidal fibres to the pontine grey demonstrated by a new application of the fluorescent fibre labelling technique. Brain Res. 1986;365:211–27. doi: 10.1016/0006-8993(86)91632-x. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of trans-cranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–9. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–8. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav Brain Res. 1998;93:167–83. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters’ nucleus and medial longitudinal fasciculus on neck, fore-limb, and hindlimb motoneurons. J Neurophysiol. 1969;32:743–58. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Zarzecki P, Schor RH, Isu N, Rose PK, Sato H, et al. Cortical influences on the vestibular nuclei of the cat. Exp Brain Res. 1999;125:1–13. doi: 10.1007/s002210050651. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476–506. doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–85. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]
- Yelnik A, Bonan I, Debray M, Lo E, Gelbert F, Bussel B. Changes in the execution of a complex manual task after ipsilateral ischemic cerebral hemispheric stroke. Arch Phys Med Rehabil. 1996;77:806–10. doi: 10.1016/s0003-9993(96)90261-0. [DOI] [PubMed] [Google Scholar]


