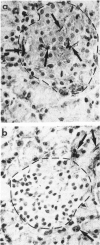
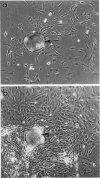
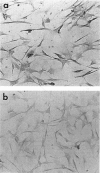
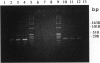
Abstract
We have succeeded in obtaining cultures of pure rat islet capillary endothelial cells. These multiply in vitro and exhibit the same antigenic phenotype as expressed in situ: von Willebrand factorhigh, Ox43 (rat endothelial marker)weak, and Ox2 (thymocyte and brain endothelium marker)high. This phenotype differs from both exocrine endothelium stained in situ and rat aorta endothelial cells cultured in vitro under identical conditions. Islet and aorta endothelial cells were cultured in the presence of various glucose concentrations. Nitrite and citrulline concentrations in culture supernatants were measured as an indirect quantification of nitric oxide formation. In islet endothelia, both nitrite and citrulline levels were found to be strongly glucose-dependent, with high levels at high glucose concentrations and vice versa, in contrast to aorta endothelial cells, where no glucose effect was found. Shifting islet endothelial cultures from high to low glucose levels or the reverse led to a slow decrease or increase in nitrite and citrulline formation with several cell generations needed to reach steady levels. Adding a combination of the cytokines interleukin-1 beta, tumor necrosis factor-alpha, and interferon-gamma to both endothelial cell cultures led to a dramatic increase of nitric oxide formation. Again with islet but not with aorta endothelial cells a modulating effect by glucose concentrations was found. Reverse-transcription-polymerase chain reaction with specific primers demonstrated the presence of constitutively expressed nitric oxide synthase-RNA in the islet capillary endothelial cells and confirmed the glucose effect. In addition, we found that cytokines indeed induce the expression of inducible synthase messenger RNA in both endothelial cells, which was not found in the absence of cytokines. Electron paramagnetic resonance spectroscopy of islet endothelial cells confirmed intracellular synthesis of nitric oxide in the presence of cytokines. In conclusion, we here for the first time provide evidence that constitutive nitric oxide synthase is also expressed in capillary endothelium and that cytokine challenge leads to the expression of the inducible isoform in these cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appels B., Burkart V., Kantwerk-Funke G., Funda J., Kolb-Bachofen V., Kolb H. Spontaneous cytotoxicity of macrophages against pancreatic islet cells. J Immunol. 1989 Jun 1;142(11):3803–3808. [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986 Jun 20;232(4757):1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- Bergmann L., Kröncke K. D., Suschek C., Kolb H., Kolb-Bachofern V. Cytotoxic action of IL-1 beta against pancreatic islets is mediated via nitric oxide formation and is inhibited by NG-monomethyl-L-arginine. FEBS Lett. 1992 Mar 24;299(1):103–106. doi: 10.1016/0014-5793(92)80110-3. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982 Oct;31(10):883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- Boyde T. R., Rahmatullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem. 1980 Sep 15;107(2):424–431. doi: 10.1016/0003-2697(80)90404-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, McDaniel M. L. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992 Dec;90(6):2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Fédérici C., Créminon C., Foignant-Chaverot N., Roux F., Claire M., Strosberg A. D., Couraud P. O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993 Apr;155(1):104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanenberg H., Kolb-Bachofen V., Kantwerk-Funke G., Kolb H. Macrophage infiltration precedes and is a prerequisite for lymphocytic insulitis in pancreatic islets of pre-diabetic BB rats. Diabetologia. 1989 Feb;32(2):126–134. doi: 10.1007/BF00505185. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kolb-Bachofen V., Epstein S., Kiesel U., Kolb H. Low-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988 Jan;37(1):21–27. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Type 1 (insulin-dependent) diabetes mellitus and nitric oxide. Diabetologia. 1992 Aug;35(8):796–797. doi: 10.1007/BF00429103. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Funda J., Berschick B., Kolb H., Kolb-Bachofen V. Macrophage cytotoxicity towards isolated rat islet cells: neither lysis nor its protection by nicotinamide are beta-cell specific. Diabetologia. 1991 Apr;34(4):232–238. doi: 10.1007/BF00405081. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Kolb-Bachofen V., Berschick B., Burkart V., Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991 Mar 29;175(3):752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Rodriguez M. L., Kolb H., Kolb-Bachofen V. Cytotoxicity of activated rat macrophages against syngeneic islet cells is arginine-dependent, correlates with citrulline and nitrite concentrations and is identical to lysis by the nitric oxide donor nitroprusside. Diabetologia. 1993 Jan;36(1):17–24. doi: 10.1007/BF00399088. [DOI] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Michel T., Collins T., Brenner B. M., Marsden P. A. Effects of interferon-gamma on nitric oxide synthase activity and endothelin-1 production by vascular endothelial cells. J Clin Invest. 1992 Sep;90(3):879–887. doi: 10.1172/JCI115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. U., Amano K., Yoon J. W. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988 Jul;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Schappert K. T., Chen H. S., Flowers M., Sundell C. L., Wilcox J. N., Lamas S., Michel T. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992 Aug 3;307(3):287–293. doi: 10.1016/0014-5793(92)80697-f. [DOI] [PubMed] [Google Scholar]
- McGuire P. G., Orkin R. W. Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest. 1987 Jul;57(1):94–105. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pukel C., Baquerizo H., Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988 Jan;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. MRC OX-43: a monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology. 1986 Feb;57(2):231–237. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Southern C., Schulster D., Green I. C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990 Dec 10;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- Suschek C., Rothe H., Fehsel K., Enczmann J., Kolb-Bachofen V. Induction of a macrophage-like nitric oxide synthase in cultured rat aortic endothelial cells. IL-1 beta-mediated induction regulated by tumor necrosis factor-alpha and IFN-gamma. J Immunol. 1993 Sep 15;151(6):3283–3291. [PubMed] [Google Scholar]
- Svensson A. M., Jansson L., Hellerström C. The volume and area of the capillaries in the endocrine and exocrine pancreas of the rat. Histochemistry. 1988;90(1):43–46. doi: 10.1007/BF00495705. [DOI] [PubMed] [Google Scholar]
- Welsh N., Eizirik D. L., Bendtzen K., Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991 Dec;129(6):3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]
- Welsh N., Sandler S. Interleukin-1 beta induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochem Biophys Res Commun. 1992 Jan 15;182(1):333–340. doi: 10.1016/s0006-291x(05)80149-4. [DOI] [PubMed] [Google Scholar]
- Wogensen L. D., Kolb-Bachofen V., Christensen P., Dinarello C. A., Mandrup-Poulsen T., Martin S., Nerup J. Functional and morphological effects of interleukin-1 beta on the perfused rat pancreas. Diabetologia. 1990 Jan;33(1):15–23. doi: 10.1007/BF00586456. [DOI] [PubMed] [Google Scholar]
- Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. Vascular smooth muscle-derived relaxing factor (MDRF) and its close similarity to nitric oxide. Biochem Biophys Res Commun. 1990 Jul 16;170(1):80–88. doi: 10.1016/0006-291x(90)91243-l. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]