Abstract
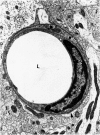
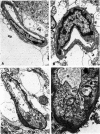
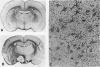
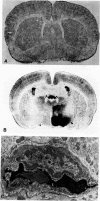
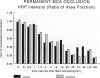
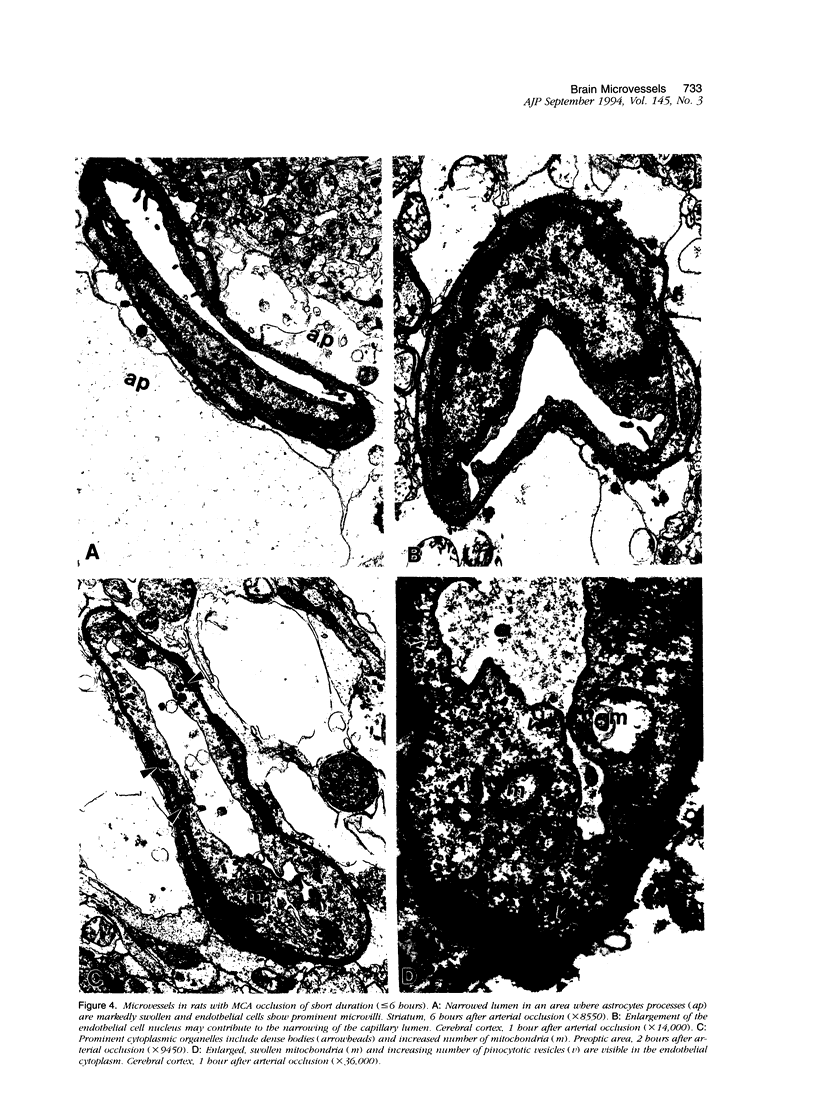
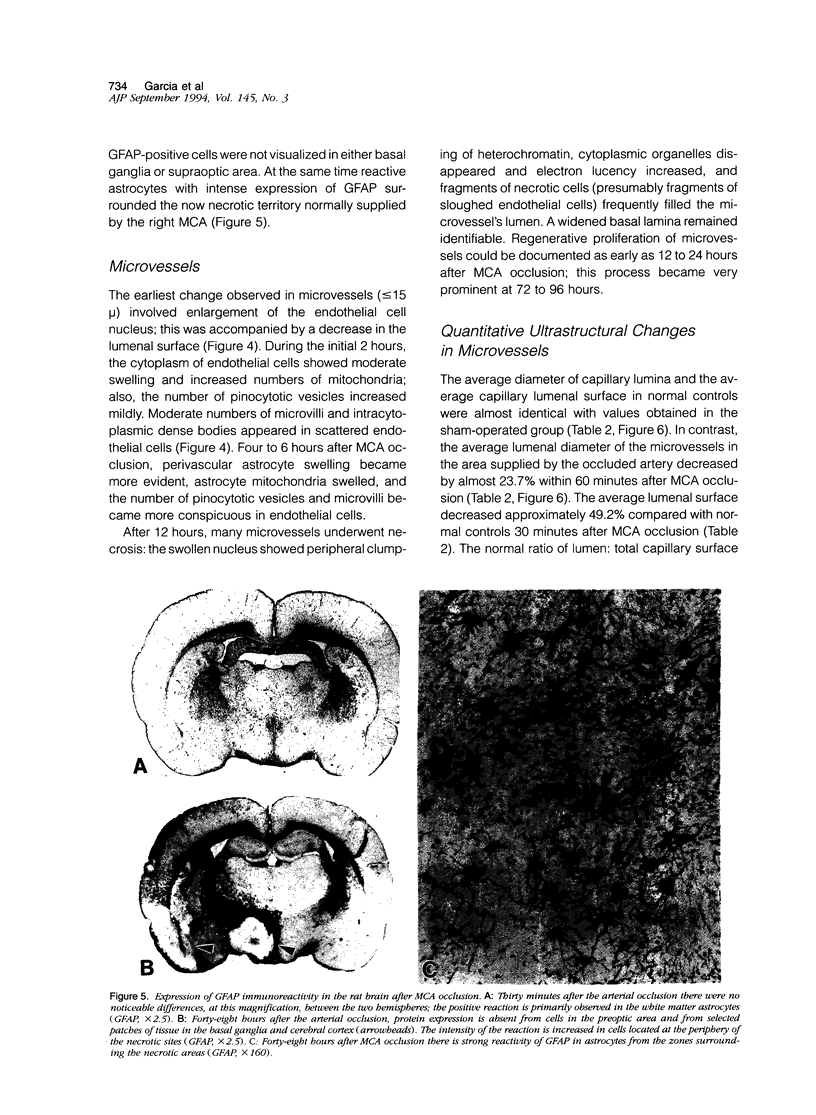
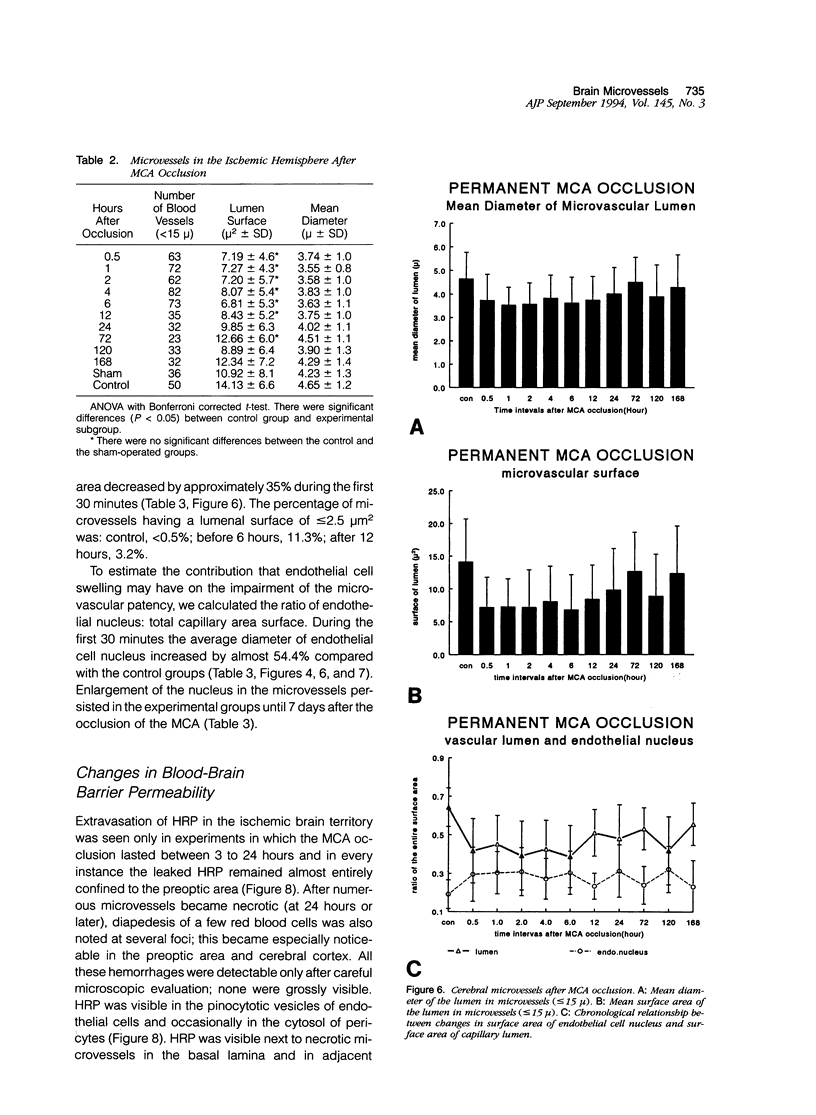
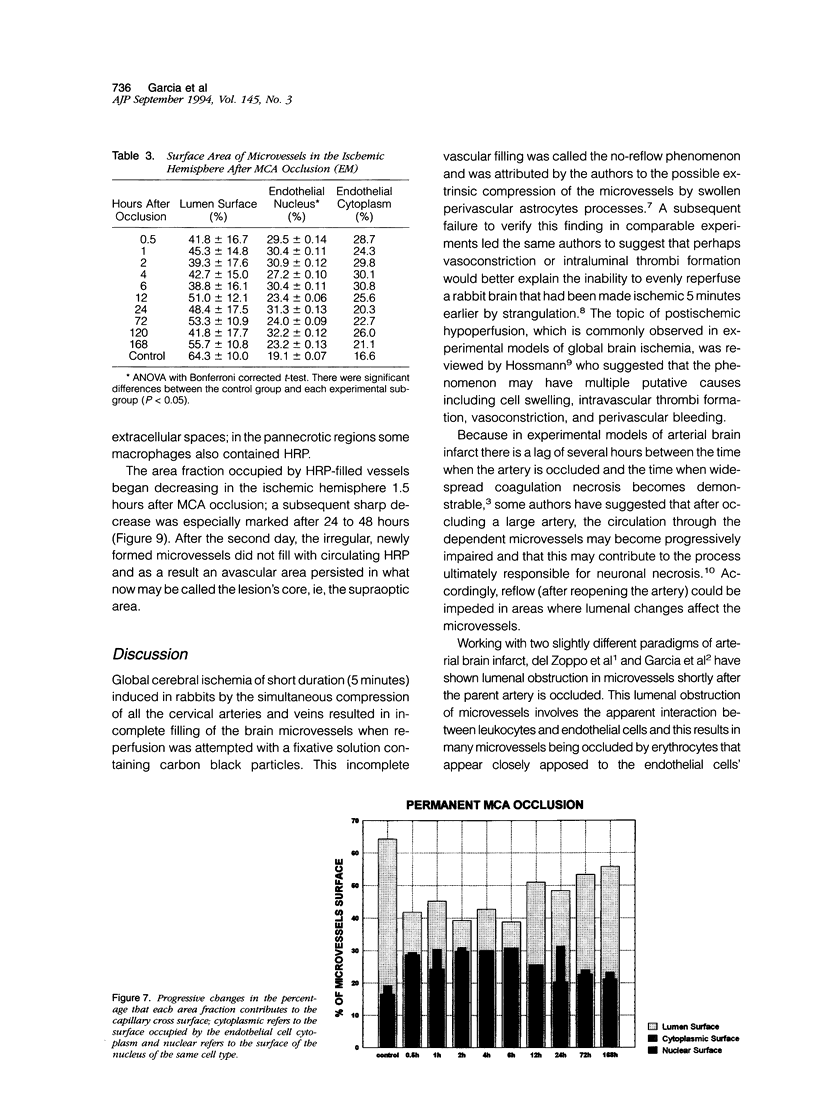
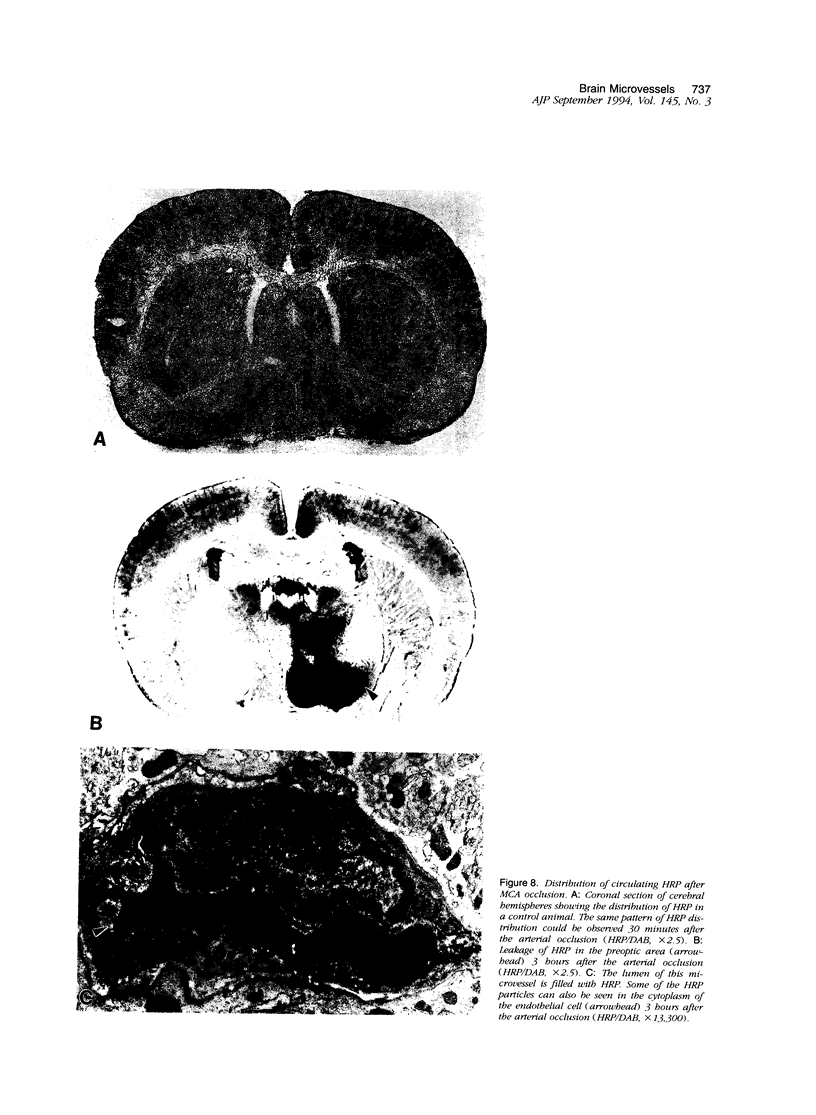
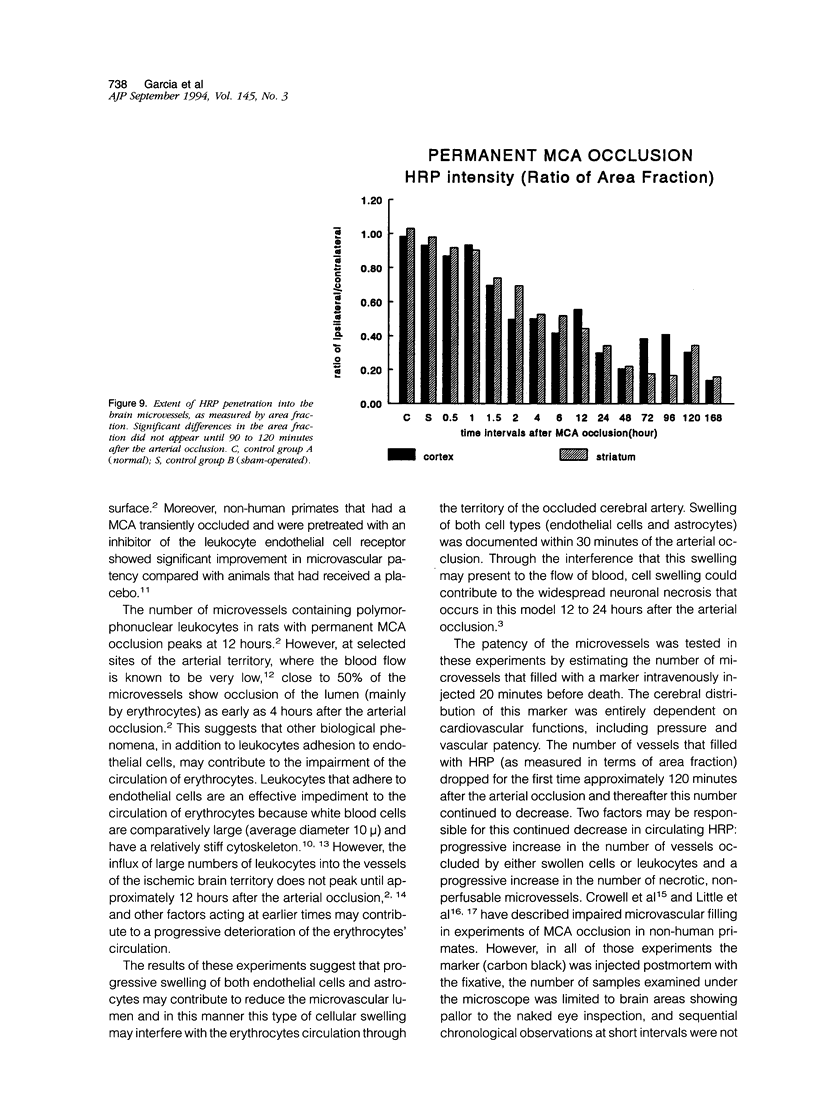
The progression from ischemic injury to pannecrosis that occurs in the rat brain several hours after occluding a large artery may be partly attributable to a worsening of the circulation through the microvessels. The objective of this study was to quantitate selected structural changes involving astrocytes and endothelial cells within an area of focal brain ischemia created by the occlusion of a middle cerebral artery. The magnitude of these structural changes was correlated with alterations in the patency to a circulating macromolecule through the microvessels (< or = 15 mu in diameter) located within the territory of the occluded artery. One hundred eighty-five adult male Wistar rats had the right middle cerebral artery occluded after threading a nylon monofilament through the external carotid artery. Experiments were terminated by either cardiovascular perfusion or decapitation and immersion fixation at intervals ranging between 30 minutes and 7 days after the arterial occlusion. Randomly selected animals from each experimental subgroup were injected intravenously with horseradish peroxidase (molecular weight 44 kd) approximately 20 minutes before death. The progressive decline in the area fraction comprised by the vessels filled with horseradish peroxidase was preceded at 30 to 60 minutes by an increase in the surface area occupied (on a cross-section of a microvessel) by endothelial cells (both nucleus and cytoplasm). This was followed by an increase of 23.7% in the mean diameter of astrocytes nuclei and a decrease of approximately 35% in lumenal surface of the microvessels. These observations suggest that the occlusion of a large cerebral artery causes prompt swelling of endothelial cells and astrocytes; both of these early biological responses may interfere with erythrocyte circulation and oxygen delivery, which (after the arterial occlusion) are entirely dependent on the circulation provided by the collateral arterial connections. Through its interference with microvascular patency and oxygen delivery, cell swelling may influence the rate at which neurons become necrotic. In this model of brain infarct the number of necrotic neurons peaks approximately 72 hours after middle cerebral artery occlusion.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
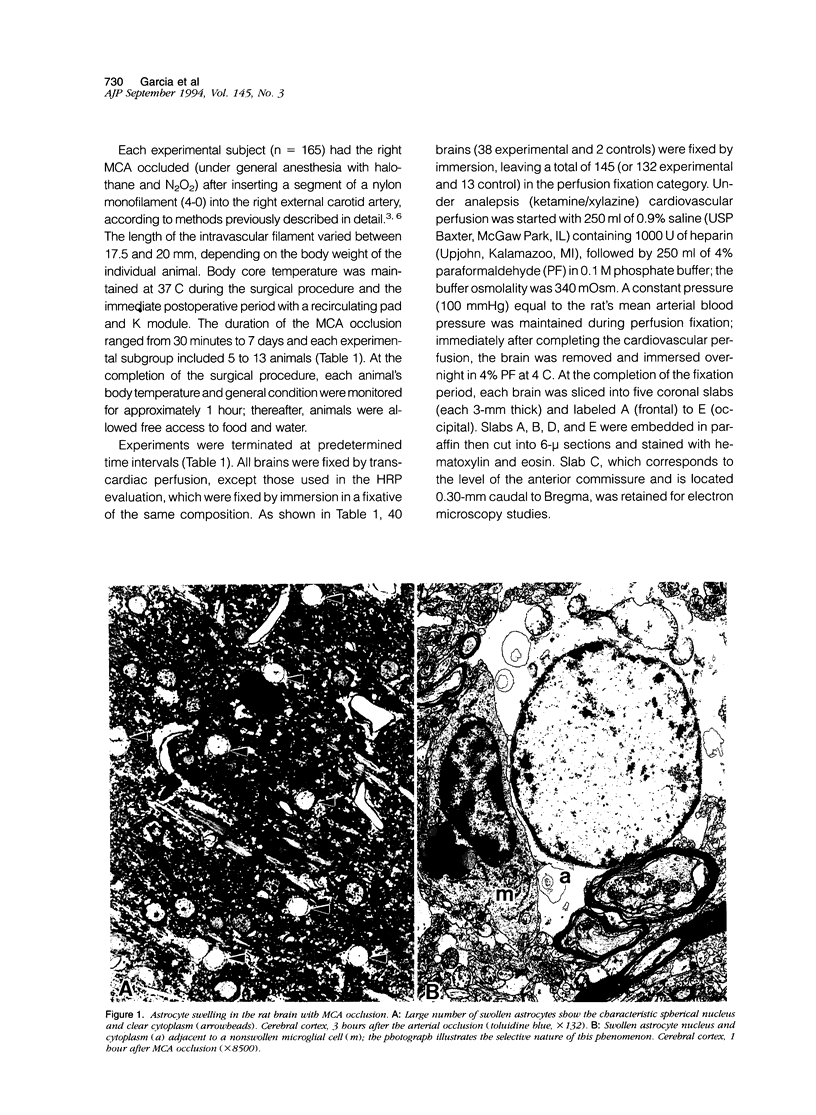

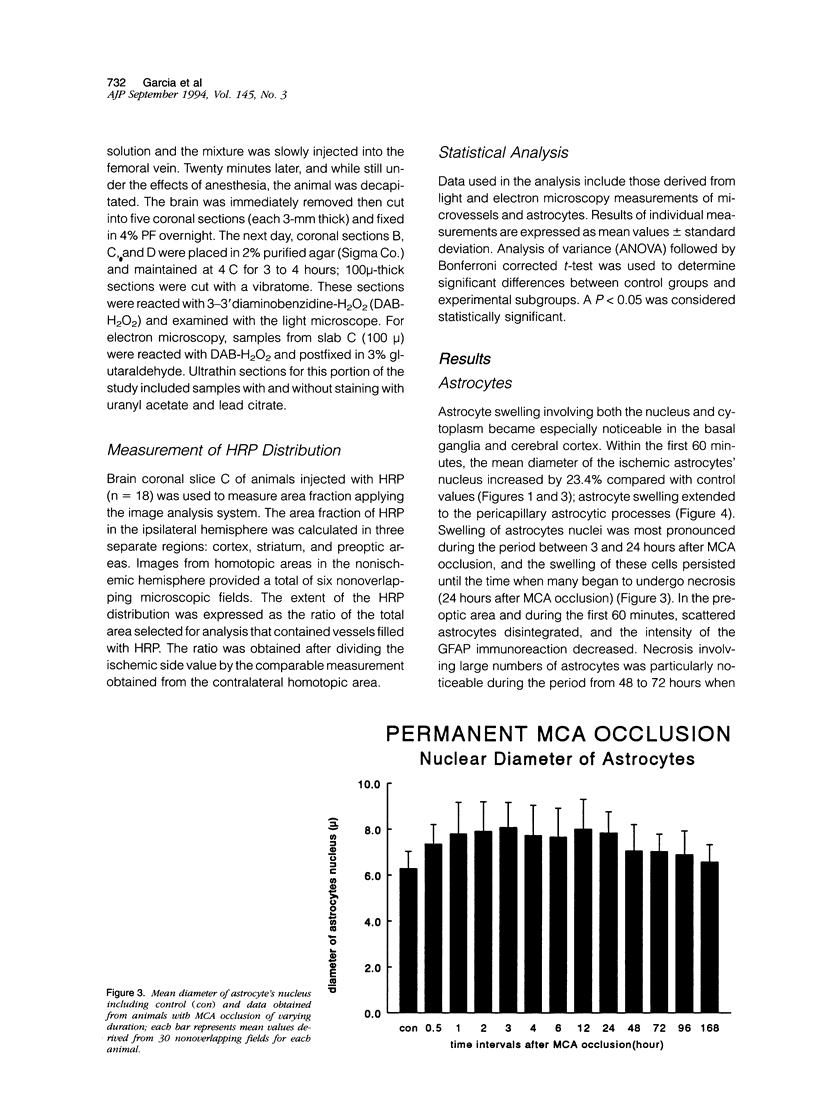


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- Astrup J., Symon L., Branston N. M., Lassen N. A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977 Jan-Feb;8(1):51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Crowell R. M., Olsson Y. Impaired microvascular filling after focal cerebral ischemia in monkeys. J Neurosurg. 1972 Mar;36(3):303–309. doi: 10.3171/jns.1972.36.3.0303. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Aoyagi M., Hartmann A., Tagashira Y. Acute cerebral infarction and hypotension: an ultrastructural study. J Neuropathol Exp Neurol. 1974 Jul;33(3):400–407. doi: 10.1097/00005072-197407000-00006. [DOI] [PubMed] [Google Scholar]
- Fischer E. G., Ames A., 3rd, Hedley-Whyte E. T., O'Gorman S. Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the "no-reflow phenomenon". Stroke. 1977 Jan-Feb;8(1):36–39. doi: 10.1161/01.str.8.1.36. [DOI] [PubMed] [Google Scholar]
- Garcia J. H., Kalimo H., Kamijyo Y., Trump B. F. Cellular events during partial cerebral ischemia. I. Electron microscopy of feline cerebral cortex after middle-cerebral-artery occlusion. Virchows Arch B Cell Pathol. 1977 Nov 3;25(3):191–206. doi: 10.1007/BF02889433. [DOI] [PubMed] [Google Scholar]
- Garcia J. H., Liu K. F., Yoshida Y., Lian J., Chen S., del Zoppo G. J. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol. 1994 Jan;144(1):188–199. [PMC free article] [PubMed] [Google Scholar]
- Garcia J. H., Mitchem H. L., Briggs L., Morawetz R., Hudetz A. G., Hazelrig J. B., Halsey J. H., Jr, Conger K. A. Transient focal ischemia in subhuman primates. Neuronal injury as a function of local cerebral blood flow. J Neuropathol Exp Neurol. 1983 Jan;42(1):44–60. doi: 10.1097/00005072-198301000-00004. [DOI] [PubMed] [Google Scholar]
- Garcia J. H., Yoshida Y., Chen H., Li Y., Zhang Z. G., Lian J., Chen S., Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993 Feb;142(2):623–635. [PMC free article] [PubMed] [Google Scholar]
- Kempski O., Staub F., Jansen M., Baethmann A. Molecular mechanisms of glial cell swelling in acidosis. Adv Neurol. 1990;52:39–45. [PubMed] [Google Scholar]
- Little J. R., Kerr F. W., Sundt T. M., Jr Microcirculatory obstruction in focal cerebral ischemia. Relationship to neuronal alterations. Mayo Clin Proc. 1975 May;50(5):264–270. [PubMed] [Google Scholar]
- Longa E. Z., Weinstein P. R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989 Jan;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Mazzoni M. C., Borgström P., Intaglietta M., Arfors K. E. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ Shock. 1989 Sep;29(1):27–39. [PubMed] [Google Scholar]
- Mazzoni M. C., Intaglietta M., Cragoe E. J., Jr, Arfors K. E. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol (1985) 1992 Oct;73(4):1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- Mori E., del Zoppo G. J., Chambers J. D., Copeland B. R., Arfors K. E. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992 May;23(5):712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989 Aug;20(8):1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Noble L. J., Hall J. J., Chen S., Chan P. H. Morphologic changes in cultured astrocytes after exposure to glutamate. J Neurotrauma. 1992 Fall;9(3):255–267. doi: 10.1089/neu.1992.9.255. [DOI] [PubMed] [Google Scholar]
- Paljärvi L., Rehncrona S., Söderfeldt B., Olsson Y., Kalimo H. Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol. 1983;60(3-4):232–240. doi: 10.1007/BF00691871. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Babiak T. Early proliferative changes in astrocytes in postischemic noninfarcted rat brain. Ann Neurol. 1982 May;11(5):510–518. doi: 10.1002/ana.410110511. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Halaby I. A. Relationship between ischemia and ischemic neuronal necrosis to astrocyte expression of glial fibrillary acidic protein. Int J Dev Neurosci. 1993 Apr;11(2):239–247. doi: 10.1016/0736-5748(93)90082-o. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Pulsinelli W. A., Jacobson G., Plum F. Edema and vascular permeability in cerebral ischemia: comparison between ischemic neuronal damage and infarction. J Neuropathol Exp Neurol. 1982 Jul;41(4):423–436. doi: 10.1097/00005072-198207000-00005. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Symon L., Branston N. M., Chikovani O. Ischemic brain edema following middle cerebral artery occlusion in baboons: relationship between regional cerebral water content and blood flow at 1 to 2 hours. Stroke. 1979 Mar-Apr;10(2):184–191. doi: 10.1161/01.str.10.2.184. [DOI] [PubMed] [Google Scholar]
- del Zoppo G. J., Schmid-Schönbein G. W., Mori E., Copeland B. R., Chang C. M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991 Oct;22(10):1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]