Abstract
The hyperpolarization-activated cation current (Ih) may influence precise auditory processing by modulating resting membrane potential and cell excitability. We used electrophysiology and immunohistochemistry to investigate the properties of Ih in three auditory brainstem nuclei in mice: the anteroventral cochlear nucleus (AVCN), the medial nucleus of the trapezoid body (MNTB) and the lateral superior olive (LSO). Ih amplitude varied considerably between these cell types, with the order of magnitude LSO > AVCN > MNTB. Kinetically, Ih is faster in LSO neurons, and more active at rest, compared with AVCN and MNTB cells. The half-activation voltage is −10 mV more hyperpolarized for AVCN and MNTB cells compared with LSO neurons. HCN1 immunoreactivity strongly labelled AVCN and LSO neurons, while HCN2 staining was more diffuse in all nuclei. The HCN4 subunit displayed robust membrane staining in AVCN and MNTB cells but weak labelling of the LSO. We used a dynamic clamp, after blocking Ih, to reinsert Ih to the different cell types. Our results indicate that the native Ih for each cell type influences the resting membrane potential and can delay the generation of action potentials in response to injected current. Native Ih increases rebound depolarizations following hyperpolarizations in all cell types, and increases the likelihood of rebound action potentials (particularly in multiple-firing LSO neurons). This systematic comparison shows that Ih characteristics vary considerably between different brainstem nuclei, and that these differences significantly affect the response properties of cells within these nuclei.
Auditory brainstem neurons process and compare binaural signals that are fundamental for sound localization. The superior olivary complex (SOC) is the first area within the auditory pathway where encoding of interaural time and level disparities (ITDs and ILDs, respectively) take place. The pathway that processes ILD cues is comprised of the anteroventral cochlear nucleus (AVCN), the medial nucleus of the trapezoid body (MNTB) and the lateral superior olive (LSO). Principal cells of the LSO receive ipsilateral excitation from AVCN spherical bushy cells and are inhibited by the MNTB principal cells that relay excitatory input from the contralateral globular bushy cells of the AVCN (Oertel, 1999). The medial superior olive (MSO) is thought to be involved in ITD computation, receiving binaural excitation from globular bushy cells of the AVCN and ipsilateral inhibition from the MNTB (Brand et al. 2002).
Since neurons at different levels of the circuit are required to process signals differently (e.g. AVCN and MNTB neurons phase lock to unilateral inputs while LSO neurons integrate bilateral excitation/inhibition), their respective properties are likely to be different to achieve this. A potentially important channel type expressed in these auditory brainstem neurons is the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel family, which underlies the hyperpolarization-activated cation current, Ih (Chen, 1997; Bal & Oertel, 2000; Cuttle et al. 2001; Koch & Grothe, 2003; Santoro & Baram, 2003; Shaikh & Finlayson, 2003; Barnes-Davies et al. 2004). Ih is a mixed inward Na+–K+ current that influences the resting membrane potential and modulates excitability (Pape, 1996; Chen, 1997; Shaikh & Finlayson, 2003). Four HCN channel isoforms (HCN1–4) have been cloned (Santoro et al. 1997; Ludwig et al. 1998). These channel isoforms vary in their kinetics and modulation by cyclic-AMP (cAMP), which can alter the voltage sensitivity of HCN channels (Pape, 1996; Santoro et al. 2000; Yamada et al. 2005). Previous studies have shown that HCN1 channels activate more rapidly than HCN2 channels which are, in turn, faster than HCN4 channels. HCN2 and HCN4 channels are strongly modulated by cAMP while HCN1 channels are only weakly affected by cAMP (Santoro et al. 2000). All four channels are non-uniformly expressed throughout the mouse CNS (except for HCN3 channels which have a wide expression but at very low levels) (Ludwig et al. 1998; Moosmang et al. 1999; Santoro et al. 2000). It has been shown that AVCN, MNTB and LSO cells express Ih (Banks et al. 1993; Cuttle et al. 2001; Barnes-Davies et al. 2004; Koch et al. 2004; Leao et al. 2005b). However, a systematic comparison of Ih characteristics between these auditory nuclei has not been made.
In this study, using electrophysiology, including the dynamic clamp, and immunohistochemistry, we examined the differences in Ih properties and HCN channel expression between neurons of the AVCN, MNTB and LSO. Our data demonstrate that Ih has distinct features in different nuclei and these differences are associated with the heterogeneous expression of HCN subunits. The distinct Ih expression patterns result in differential modulation of firing properties of neurons in the auditory brainstem.
Methods
Slice preparation
Mice (CBA strain) at age 12–14 days postnatal were killed according to the Australian National University Animal Ethics Committee and Wright State University IACUC protocols. Following decapitation the brain was dissected in ice-cold standard artificial cerebral spinal fluid (ACSF) (mm: 130 NaCl, 3.0 KCl, 5.0 MgCl2, 1.25 NaH2PO4, 26.2 NaHCO3, 10 glucose, 218 sucrose equilibrated with 95% O2, 5% CO2). Transverse slices (180–200 μm) were made of the ventral cochlear nucleus (VCN), the medial nucleus of the trapezoid body (MNTB) and the lateral superior olive (LSO) using an oscillating tissue slicer (Integraslice 7550 PSDS, Campden Instruments, UK). Slices were incubated in normal ACSF (mm: 130 NaCl, 3.0 KCl, 1.3 Mg2SO4, 2.0 CaCl2, 1.25 NaH2PO4, 26.2 NaHCO3, 10 glucose, equilibrated with 95% O2, 5% CO2) at 35°C for 1 h and subsequently held at room temperature (22–24°C) or at physiological temperature (35–37°C), for electrophysiological recording.
Electrophysiological recordings
For whole-cell current- and voltage-clamp recordings one slice was transferred to a recording chamber of standard design and fixed to the glass bottom of the chamber with a nylon grid on a platinum frame. The slice chamber was constantly perfused with ACSF at a rate of 2 ml min−1. Neurons in the AVCN, MNTB or LSO were visualized using an Olympus microscope fitted with a 5 × objective or a 60 × water-immersion objective combined with infrared videomicroscopy. Patch pipettes were made from borosilicate glass capillaries (tip Φ 2 μm, Vitrex, MODULOHMA/S, Denmark) using a patch-pipette puller (Narishige PP-83, Japan). The electrode resistance was typically 3–5 MΩ. The intracellular solution for current-clamp and voltage-clamp recordings was (mm): 130 KMeSO4, 9.5 KCl, 3.0 MgCl2, 10 TES, 3.0 Mg-ATP, 0.3 GTP-tris and 0.2 EGTA). The pH was adjusted to 7.3 using KOH. The cell was held at −60 mV and the access resistance was routinely compensated by > 80%. Current and voltage steps were applied and the corresponding neuronal responses recorded using an Axopatch 200B or an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA, USA). Electrical signals were low-pass filtered at 5 kHz, digitized at 10 kHz using Axograph (Axon) and data were analysed with Matlab SV13 (Matworks, USA) software. Liquid junction potentials were not compensated for. The pipette offset was usually between 3.5 and 4.2 mV. In several experiments, 20 μm ZD7288 (Tocris Cookson Inc., UK) was added to the bath solution in order to block Ih (for 10–20 min before the recordings). Neurobiotin (0.5%) or Alexa-488 (Molecular Probes) was added to the internal solution in order to determine the spatial position of cells. We have previously demonstrated that there is a gradient of ionic currents, including Ih, in the MNTB. In this study, we have therefore used values obtained from the intermediate or central region of the MNTB as representative of an average value for the nucleus.
Dynamic clamp
We simulated Ih (and low-threshold K+ currents in some LSO cells) in neurons using a dynamic clamp. In order to assess the effect of Ih kinetics on cell function, we used two kinetically distinct Ih, a fastτIh based on our LSO recordings and a slow τIh based on our AVCN recordings in room temperature. Simulated Ih also varied in amplitude (A) (high-A Ih based on the LSO Ih amplitudes and low-A Ih based on AVCN Ih amplitudes). Our dynamic clamp method was implemented on a second computer running real-time Linux and custom-made software that read voltage and generates currents at 40 kHz. A description of the dynamic clamp technique can be found in our previous study (Leao et al. 2005b).
In order to add different macroscopic Ih to real neurons, we first blocked the ‘real’ Ih with 20 μm of ZD7288. Simulated Ih was calculated by 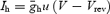 where
where 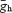 is the maximal hyperpolarization-activated conductance, u is the evolution variable, V is the membrane voltage and Vrev is the Ih reversal voltage. Evolution variables were obtained by the following equation:
is the maximal hyperpolarization-activated conductance, u is the evolution variable, V is the membrane voltage and Vrev is the Ih reversal voltage. Evolution variables were obtained by the following equation:
| (1) |
where x = u. Activation time constant versus voltage (τx(V)) and steady-state conductance versus voltage (x∞ ‘Boltzmann’) functions for Ih slowτ and Ih fastτ were, respectively:
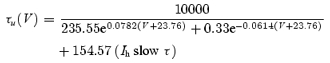 |
(2) |
| (3) |
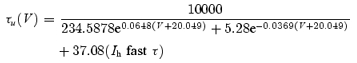 |
(2) |
| (3) |
These equations where obtained by fitting the time constants of Ih and the normalized Ih conductance across various voltages from Ih recordings of AVCN bushy cells (Ih slow τ) and LSO cells (Ih fastτ). Note, the Boltzmann relations shown here refer to steady-state conductance, not steady-state current. In order to account for the differences in amplitude found in different nuclei, we used different amplitudes of 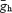 (15 nS for low amplitude and 30 nS for high amplitude – low amplitudes in MNTB neurons had
(15 nS for low amplitude and 30 nS for high amplitude – low amplitudes in MNTB neurons had 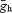 = 7 nS). We have also simulated low-threshold voltage-dependent K+ currents, ILT (Leao et al. 2005) using the dynamic clamp in some LSO cells.
= 7 nS). We have also simulated low-threshold voltage-dependent K+ currents, ILT (Leao et al. 2005) using the dynamic clamp in some LSO cells.
Immunohistochemistry
Deeply anaesthetized mice were perfused intracardially with cold saline followed by fixative solution (4% paraformaldehyde in 0.1 m phosphate buffer (PBS), pH 7.4). The brainstem was dissected and post-fixed for up to 4 h before being transferred to PBS containing 15% sucrose. Transverse sections (30 μm thick) of the brainstem were obtained at the level of the AVCN and the LSO, using a cryostat. Brainstem slices were then incubated overnight in one of the following primary antibodies: anti-HCN1, anti-HCN2 or anti-HCN4 (Alamone Laboratories, rabbit, 1 : 100) followed by incubation (1–3 h) in the appropriate secondary antibody conjugated to FITC or Cy3 (Jackson, 1 : 50). Sections were mounted on glass slides and cover-slipped with fluorescent mounting medium (Vectashield, Vector labs).
Glutamatergic synaptic terminals were visualized with anti-VGlut1 (Chemicon, guinea pig, 1 : 1000). Fluorescent images were collected using a laser scanning confocal microscope (Olympus Fluoview) with 20 ×, 40 × or 60 × oil immersion objectives, at 1024 pixels by 1024 pixels resolution.
Image capture and quantity analysis
HCN1-immuno reactivity (IR) was examined with bright field illumination using a Nikon labophot-2 research microscope, equipped with a 20 × and a 60 × objective. Images were captured with a Spot CCD colour video camera; model RT Slider (Diagnostic Instruments, Sterling Heights, MI, USA). The resultant 8-bit monochrome image contained a greyscale of pixel intensities from 0 to 255, with 0 representing white and 255 representing black. Intensity analysis of immunoreactivity was performed in eight coronal sections throughout the rostrocaudal dimension of each nucleus. Optical density of HCN1-IR was measured by manually outline the staining surrounding cells using ImagePro Plus software (Media Cybernetics Inc.).
Data analysis
Results are presented as mean ± standard error of the mean (s.e.m.). Statistical calculations were preformed using Student's t tests, comparing samples with equal variance, or a single-factor ANOVA test, and significance (*) was noted if P < 0.05 and (**) for P < 0.01 (two-tailed), unless otherwise mentioned in the text.
Results
Whole-cell patch-clamp recordings were made from visualized neurons in the AVCN, MNTB and LSO in CBA mice age 12–14 days postnatal. Bushy cells in the AVCN were identified by their appearance under the microscope and onset-firing pattern in response to depolarizing current injections, and confirmed morphologically using a fluorescent dye in the internal solution (Alexa 488). Passive membrane properties of cells were measured before and after block of the hyperpolarization-activated channels with ZD7288, and are shown in Table 1. To gain additional insight into the consequences of different kinetics and amplitudes of Ih we have subsequently used the measured parameters of Ih in different combinations and applied them using the dynamic clamp to AVCN, MNTB and LSO cell types.
Table 1.
Passive membrane properties of auditory neurons before and after ZD7288
| AVCN | MNTB | LSO | |
|---|---|---|---|
| Resting potential (mV) | −57.1 ± 0.8 | −62.6 ± 0.8 | −56.5 ± 0.6 |
| + ZD7288 | −59.6 ± 2.9 | −63.2 ± 0.7 | −66.1 ± 3.3 |
| Input resistance (MΩ) | 120.8 ± 6.3 | 228.2 ± 14.0 | 70.1 ± 5.3 |
| + ZD7288 | 170.6 ± 15.6 | 257.2 ± 18.5 | 111.4 ± 13.0 |
| Capacitance (pF) | 22.3 ± 1.1 | 24.1 ± 1.1 | 22.2 ± 1.2 |
| Control (n) | 23 | 7 | 30 |
| + ZD7288 (n) | 9 | 7 | 6 |
Neurons in the AVCN, MNTB and LSO differ in their Ih expression
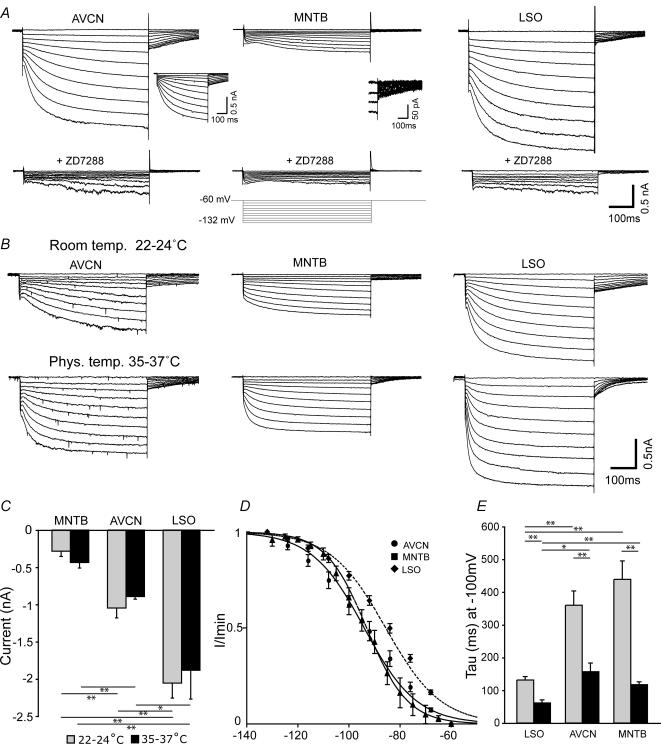
Activation of Ih was examined by injecting negative voltage steps (−60 to −132 mV, 8 mV increments) for 500 ms. Ih amplitudes were measured as the peak current amplitude subtracted by the peak current response following bath application of 20 μm ZD7288 (Fig. 1A and C). Longer voltage steps (1 or 3 s) did not significantly increase Ih amplitudes (data not shown), although these longer voltage steps were not used routinely because they caused electrophysiological recording instability. Both the amplitude and kinetics of Ih were quite variable in the AVCN, as emphasized by the inset in Fig. 1A which shows a second example trace with slower kinetics.
Figure 1. Neurons in the AVCN, MNTB and LSO exhibit diverse Ih expression.
A, upper panel shows example current traces from an AVCN bushy cell, a MNTB principal cell and a LSO principal cell. The cells represent the whole-cell current activated by negative voltage steps (10 steps from −60 to −132 mV in 8 mV increments, during 500 ms, see lower inset). Lower panel shows current traces from the same cells as above after application of 20 μm ZD7288 (same voltage steps as above) (scale bars: 0.5 nA, 100 ms, room temperature). The capacitance artefacts have been subtracted from the beginning of traces. Inset left: current trace showing an example with slower kinetics for an AVCN cell (scale bars: 0.5 nA, 100 ms). Inset centre: tail currents of the MNTB cells on a magnified scale (scale bars: 50 pA, 100 ms) B, upper panel show example current traces from an AVCN bushy cell, a MNTB principal cell and a LSO principal cell recorded in room temperature (22–24°C) after application of negative voltage steps (10 steps from −60 to −132 mV, during 500 ms). Lower panel shows current traces from the same cells, using the same voltage steps as above, after the bath temperature was heated to physiological temperature (35–37°C). The capacitance artefacts have been subtracted from the beginning of traces. (scale bars: 0.2 nA, 100 ms). C, summary of the ZD7288-sensitive Ih amplitude in room temperature (RT, grey bars) and physiological temperature (PT, black bars) (current activated by negative voltage steps subtracted by the current recorded after application of ZD7288, in response to a negative voltage step at −132 mV, 500 ms) in bushy cells of the AVCN (RT, n = 7; PT, n = 4) and principal neurons of the MNTB (RT, n = 12; PT, n = 3) and LSO (RT, n = 21; PT, n = 4). Error bars represent s.e.m. with P < 0.01 (**) or P < 0.05 (*). D, AVCN (•, line, n = 17), MNTB (▴, line, n = 13) and LSO (♦, dashed line, n = 17) tail currents were fitted to a Boltzmann equation to yield the Ih voltage dependency. Error bars show s.e.m.E, summary of the activation time constant (tau) at −100 mV, when a single exponential function fitted well to all the cells current response at room temperature (RT, grey bars) and physiological temperature (PT, black bars), of bushy cells of the AVCN (RT, n = 9; PT, n = 4) and principal neurons of the MNTB (RT, n = 12; PT, n = 3) and the LSO (RT, n = 15; PT, n = 4). Error bars show s.e.m., P < 0.01 (**) or P < 0.05 (*).
The ZD7288-sensitive current amplitudes activated by negative voltage steps (−60 to −132 mV) were 1.05 ± 0.13 nA in AVCN cells (n = 7), 0.28 ± 0.07 nA in MNTB cells (n = 12) and 2.06 ± 0.20 nA for LSO cells (n = 21) and were significantly different (P < 0.01, ANOVA). Since LSO principal cells can be single firing (SF, cells that fire single action potentials in response to sustained current injections) and multiple firing (MF, cells that fire multiple action potentials in response to sustained current injections) we compared Ih in single and multiple firing LSO neurons. There was no significant difference in ZD7288-sensitive current between SF cells (−1.98 ± 0.26 nA, n = 9) and MF cells (−2.17 ± 0.39 nA, n = 9) in response to negative voltage steps (−60 to −132 mV), in agreement with Barnes-Davies et al. (2004). Heating the slice chamber to 35–37°C gave a ZD7288-sensitive current of 0.89 ± 0.03 nA for AVCN bushy cells (n = 4), 0.43 ± 0.07 nA for MNTB cells (n = 3) and 1.88 ± 0.39 nA for LSO cells (n = 4). There was no difference in maximal Ih amplitude at room temperature and physiological recording temperature.
Tail currents elicited by the voltage protocol shown previously (−60 to −132 mV) in AVCN bushy cells (n = 17), MNTB principal cells (n = 9) and LSO principal cells (n = 17) were analysed by fitting a Boltzmann function (I/Imin = [1 + exp (V1/2 − V)/k]−1, where V1/2 is the half-activation voltage and k is the slope factor) to the normalized current versus voltage relationships. There was a significant difference in V1/2 (P = 0.002) between AVCN (V1/2 = −94.0 ± 2.2 mV) and LSO cells (V1/2 = −85.6 ± 1.2 mV), as well as a significant difference (P = 0.003) between LSO and MNTB cells (V1/2 = −93.0 ± 2.2 mV). There was no difference in V1/2 between AVCN and MNTB cells. Instead, the slope factor was different between AVCN (k = 10.3 ± 0.5) and MNTB (k = 8.0 ± 0.4) neurons (P = 0.01), and between MNTB and LSO (k = 10.8 ± 0.4) cells (P = 0.0001) (Fig. 1D).
The activation time constant (τ) was measured by fitting a single exponential function to the currents produced by hyperpolarizing voltage steps. At room temperature, the fit of a single exponential equation to the current trace after a −100 mV voltage step gave a τ for AVCN bushy cells of 360 ± 43.5 ms (n = 9), τ = 438.6 ± 56.3 ms for MNTB principal cells (n = 12) and τ = 131.1 ± 10.8 ms for LSO principal cells (n = 15) (Fig. 1E). These values were significantly different between AVCN, MNTB and LSO cells (P < 0.0001, ANOVA). At voltages to −100 mV, single exponential functions provided good fits to the currents in the three nuclei. In AVCN and LSO cells, τ was better fitted by double exponentials for more hyperpolarized potentials (< −100 mV). However, for comparisons between the three nuclei, we used data in which τ was fitted with single exponentials. Tau values were also significantly different between the nuclei at physiological temperature (P = 0.016, ANOVA). Tau decreased to 156.5 ± 26.7 ms for AVCN bushy cells (n = 4), 117.2 ± 8.5 ms for MNTB cells (n = 3) and 61.2 ± 9.6 ms for LSO cells (n = 4) after a single exponential fit, although a double exponential fit was better for LSO traces at physiological temperature at −100 mV (τfast = 22.2 ± 3.7 ms and τslow = 168.2 ± 38.5 ms). All cells had significantly smaller tau values at 35–37°C compared with at room temperature (P < 0.01 for all, see Fig. 1E).
HCN channel subunits are differently expressed in the three nuclei
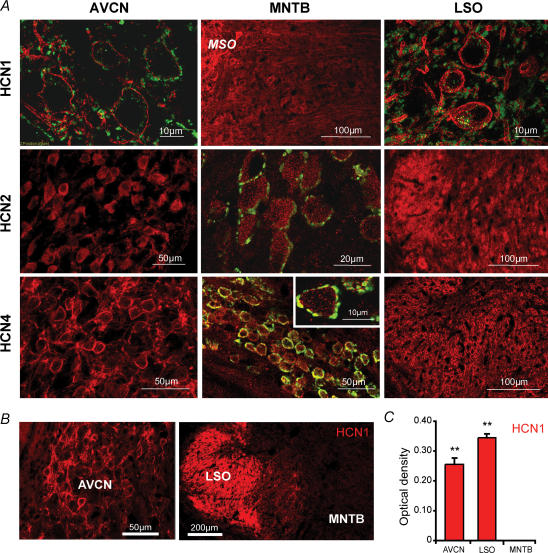
Immunohistochemistry using polyclonal antibodies against HCN1, HCN2 and HCN4 subunits revealed differential expression of these channels across the three brainstem nuclei (Fig. 2). Neuronal cell bodies in the LSO and AVCN exhibit strong membrane expression of HCN1 protein, whereas MNTB neurons express HCN1 at low levels. In the AVCN, membrane labelling appeared to be primarily in postsynaptic membranes of bushy cells, including some proximal processes. Most of the HCN1 labelling in the MNTB was co-localized with anti-VGlut1 labelling, which is a marker for glutamatergic presynaptic terminals, thus suggesting a presynaptic localization in calyceal terminals. HCN1 was highly expressed in LSO principal cells, with very strong somatic and dendritic membrane labelling. In the AVCN and LSO, the dense cell surface labelling was composed of fine, uniformly distributed punctae seen by high magnification (Fig. 2A, top row).
Figure 2. Composition of HCN family proteins in the AVCN, MNTB and LSO.
A, all red staining represents anti HCN1, -2 or -4 labelling (see row label for subunit). Green labelling, shown in high magnification images and MNTB nucleus outline, represents presynaptic terminals as stained with anti-vesicular glutamate transporter 1 (VGlut1). The HCN1 antibody showed strong membrane labelling of somata and processes in the AVCN and the LSO but only weak staining was observed in the MNTB (by comparison, some HCN1 staining of the MSO can be seen to the left of the MNTB). The HCN2 antibody labelled all nuclei diffusely, with some somatic and some surface labelling in the AVCN. The MNTB showed the highest levels of HCN2 expression of the three nuclei. The HCN4 antibody labelled membranes of neurons in the AVCN and LSO nicely. The MNTB also expressed some HCN4 labelling that was co-localized with VGlut1, showing a probable presynaptic labelling (see inset). B, fluorescent images of brainstem slices shows strong anti-HCN1 labelling (red) in the LSO, while the AVCN (magnification of the dorsal part) have robust staining compared with the MNTB showing very weak immunoreactivity (scale bars: 50 μm (left panel), 200 μm (right panel). C, optical density measurements of HCN1 immunoreactivity within neurons in the AVCN, LSO and MNTB. Significance (**) represents P < 0.01, paired Student's t test, error bars represent s.d.
AVCN cells displayed moderate cytoplasmic HCN2 staining with apparently little membrane labelling. There was also some indication of presynaptic HCN2 labelling in the AVCN. The MNTB showed moderate HCN2 labelling in the cytoplasm of principal neurons, and there was no evidence of presynaptic HCN2 staining in MNTB sections. LSO cells displayed a widespread but diffuse staining of HCN2 (Fig. 2A, middle row).
AVCN cells showed a significant expression of HCN4 in bushy cell surface membranes. The MNTB exhibits a tonotopic gradient of postsynaptic HCN4 expression in principal neurons, with higher HCN4 labelling in medial versus lateral regions (Leao et al. 2006); there appears to be minimal presynaptic expression of HCN4 in the MNTB. The LSO showed weak HCN4 expression in the membrane, although not in every cell (Fig. 2A, bottom row). A noticeable difference in strength of HCN1 expression was seen between the AVCN, LSO and MNTB nuclei (Fig. 2B). The mean optical density of immunoreactivity against HCN1 was significantly different (P < 0.01) between nuclei in the AVCN (0.255 ± 0.023), LSO (0.344 ± 0.013) and the MNTB, where staining was too weak to identify cell boundaries (Fig. 2C; Student's paired t test, errors represent standard deviation (s.d.)).
Ih modulates voltage responses to current injection
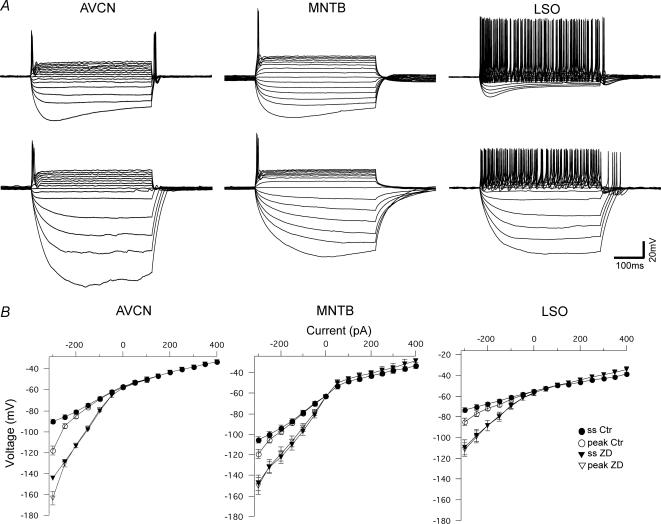
AVCN, MNTB and single firing LSO neurons responded to depolarizing current steps (50–400 pA, in 50 pA increments, 200 ms), with usually one or two action potentials (APs). The LSO principal cells also have a population of multiple firing neurons, probably due to a lower expression of low-threshold K+ currents (Barnes-Davies et al. 2004). Application of hyperpolarizing current steps (−50 to −300 pA, in 50 pA increments, 200 ms) caused a depolarizing sag (indicative of Ih activation) in neurons in all three nuclei (Fig. 3A). AVCN bushy cells showed prominent sags that slowly depolarized the membrane potential during hyperpolarization (Fig. 3A). MNTB principal cells usually showed weak depolarizing sags, also with slow depolarizations (Fig. 3A). Interestingly, LSO principal cells showed a small sag, quickly followed by depolarization (Fig. 3A). The negative peak voltage of the sag after negative current steps (−300 pA) was −118.3 ± 4.3 mV for AVCN (n = 18), −119.0 ± 4.3 mV for MNTB (n = 7) and −85.1 ± 3.9 mV for LSO cells (n = 26). Application of 20 μm ZD7288 decreased resting membrane potentials (see Table 1) and abolished depolarizing sags in all cell types (negative peak voltage after ZD7288 in AVCN: −163.6 ± 6.4 mV (n = 6), MNTB: −150.1 ± 8.3 mV (n = 7) and LSO: −111.3 ± 6.7 mV (n = 6)). Post-hyperpolarization action potentials (rebound APs) were also eliminated by ZD7288 (see AVCN traces in Fig. 3). However, in some cases when the hyperpolarizing currents caused the membrane voltage to descend below −150 mV (producing very unstable recordings) some multi-firing LSO cells showed action potentials after the termination of the negative current step (see LSO traces in Fig. 3A).
Figure 3. Voltage responses to depolarizing and hyperpolarizing 200 ms current step injections (−300 to +400 pA) of neurons in the AVCN, MNTB and LSO.
A, example of an onset response by an AVCN bushy cell (left), a MNTB principal neuron (middle) and a multiple firing LSO principal cell (right) before (upper panel) and after blocking of Ih with ZD7288 (lower panel). Scale bars: 100 ms and 20 mV. B, voltage–current relationships of the three neuron types measured at negative peak (peak Ctr, ^) and at the steady state (ss Ctr, •) of the voltage deflection under normal recording conditions and after application of ZD7288 (peak ZD, ◊, and ss ZD ▾). AVCN cells (n = 18) showed a wide sag and rebound action potential that was abolished by ZD7288. MNTB cells (n = 7) showed a small, wide sag and no rebound action potential. LSO cells (n = 26) showed a small, fast depolarizing sag and rebound spikes.
Current–voltage (I–V) relationships were also examined for the three cell types (Fig. 3B) by measuring peak voltages (measured at the most negative voltages) and steady-state currents (measured over the last 5 ms of the current step). All three brainstem neurons showed a flat but linear I–V relationship between depolarizing current injections (50–400 pA) and steady-state voltage, which did not change after application of ZD7288. MNTB and LSO cells showed a slight increase in voltage at positive current steps after blocking Ih. Voltage responses to negative current injections (−300 pA) showed a diversity in subtracted voltage (negative peak minus steady-state voltage) of 27.9 ± 3.5 mV, 13.3 ± 2.6 mV and 11.4 ± 2.4 mV for AVCN (n = 18), MNTB (n = 7) and LSO (n = 26) neurons (P < 0.001, ANOVA: single factor), respectively, that was not seen after ZD7288 application.
Effect of Ih on resting membrane potential and action potentials in AVCN, MNTB and LSO neurons
Using the dynamic clamp, we first altered Ih characteristics of AVCN, MNTB and LSO cells in order to assess the effect of Ih amplitude and kinetics on resting membrane potential. In order to assess whether Ih could be simulated by our dynamic clamp we compared control conditions before application of ZD7288 with the appropriate dynamic clamp simulation (Ih slowτ/lowA for AVCN and MNTB cells and Ih fastτ/highA for LSO cells – with maximal Ih conductance of 15 ± 1, 7 ± 1 and 30 ± 2 nS, for AVCN, MNTB and LSO, respectively). If a similar membrane potential was reached after Ih was blocked pharmacologically and subsequently reintroduced as the simulated Ih, the cell was accepted for further dynamic clamp experiments in which Ih was altered.
The resting membrane potential of AVCN bushy cells (n = 4) in control conditions, before application of ZD7288, was −58.8 ± 1.7 mV and after blocking Ih was −65.4 ± 5.0 mV. After reintroduction of an Ih appropriate for AVCN bushy cells (Ih slow τ/lowA) the membrane potential returned to −60.4 ± 0.4 mV. Increasing Ih amplitude (Ih slow τ/highA) caused the cell to depolarize approximately 1 mV (to −57.7 ± 1.7 mV, P = 0.04, paired t test). Adding a fast Ih to the membrane caused further depolarization (to −56.1 ± 1.7 mV for Ih fastτ/lowA and −53.7 ± 1.4 mV for Ih fastτ/highA, P < 0.01 for both cases, paired t test). In MNTB principal neurons (n = 4), altering only Ih amplitude (Ih slowτ/highA) caused the cell membrane to depolarize by 3 mV (from −65.3 ± 0.8 to −61.7 ± 0.2 mV, P = 0.01, paired t test). Addition of fast Ih also caused a significant change in cell membrane resting potential (to −63.5 ± 0.6 mV, Ih fastτ/lowA, P = 0.02, and to −61.4 ± 0.5 mV, Ih fastτ/highA, P < 0.01, paired t test). In LSO neurons (SF, n = 5), simulating Ih with different kinetics and amplitude dramatically altered the resting potential of the cells (from −61.3 ± 0.8 to −65.7 ± 0.4 mV, −64.6 ± 0.5 mV and −67.1 ± 0.2 mV for Ih slowτ/highA, Ih fastτ/lowA and Ih slowτ/lowA, respectively; P < 0.01 for all cases, n = 5; paired t test).
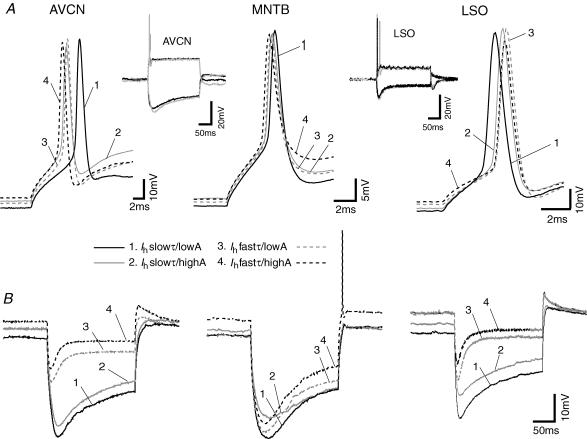
Action potential properties (amplitude and half-width) were also differently affected by changes in Ih in different nuclei. Using a 200 pA current step, control AP amplitude in AVCN cells was equal to 62.8 ± 5.3 mV and did not change significantly by altering Ih amplitude (Ih slowτ/highA). Changes in Ih kinetics significantly decreased the AP amplitude (58.7 ± 5.4 mV for Ih fastτ/lowA and 57.5 ± 5.9 mV for Ih fastτ/highA, P = 0.01 in both cases, n = 4, paired t test; Fig. 4A). AP amplitude in MNTB neurons was only affected by differences in Ih amplitude (control: 61.5 ± 4.1 mV; Ih slowτ/highA: 60.2 ± 3.9 mV; Ih fastτ/highA: 56.2 ± 3.1 mV; P = 0.03 and 0.01, respectively, n = 5, paired t test; Fig. 4A). In LSO neurons, ‘slowing’ Ih increased AP amplitude (control: 91.4 ± 5.7 mV; Ih slowτ/highA: 96.5 ± 3.4 mV; Ih slowτ/lowA: 99.8 ± 4.2 mV; P = 0.02 and 0.04, respectively, n = 5, paired t test; Fig. 4A). Action potential half-width in AVCN bushy cells was also affected by changes in Ih amplitude and kinetics (control: 1.30 ± 0.05 ms; Ih slowτ/highA: 1.13 ± 0.02 ms; Ih fastτ/lowA: 1.18 ± 0.01 ms; Ih fastτ/highA: 1.16 ± 0.02 ms; P < 0.01, P = 0.04, P = 0.03, respectively, n = 4, paired t test). In MNTB and LSO neurons, AP half-width was not affected by changes in Ih properties.
Figure 4. Ih differently affect membrane properties and AP characteristics in AVCN and SOC neurons.
A. examples of APs in response to a 200 pA current step using different Ih amplitudes and kinetics simulated by dynamic clamp in an AVCN bushy cell, a MNTB principal neuron and a LSO principal neuron. The continuous black traces show the neuron response with a slow τ and low-amplitude Ih (Ih slowτ/lowA is the control condition for AVCN bushy cells and MNTB neurons); continuous grey traces show the cell response when a slow τ and high-amplitude Ih was simulated (Ih slowτ/highA); dashed black traces represent cell responses to depolarizing current step when a fastτ and high-amplitude Ih was added to the membrane (Ih fastτ/highA is the control condition for LSO neurons) and the dashed grey trace show the cell response to a simulated fastτ and low-amplitude Ih (Ih fastτ/lowA). Insets, left, AVCN neuron response to a 200 pA and a −300 pA current step under control conditions (black traces) and with a simulated control conditions (Ih slowτ/lowA, grey traces); right, LSO neuron response to a 200 pA and a −300 pA current step under control conditions (black traces) and with a simulated control condition (Ih fastτ/highA, grey traces). B, AVCN, MNTB and LSO neuron responses to a −300 pA current step with different Ih properties as in A (all 3 panels have the same scale).
Ih is known to contribute to AP delay (time between 5% maximum AP amplitude and peak) in MNTB cells (Leao et al. 2005a); we thus investigated the effect of different Ih properties on AP delay. In AVCN bushy cells, neither Ih amplitude nor kinetics altered AP delay (for 200 pA current steps; Fig. 4A). MNTB neurons were as expected affected by changes in Ih (control: 3.1 ± 0.1 ms; Ih slowτ/highA: 2.7 ± 0.2 ms, P = 0.01; Ih fastτ/lowA: 2.6 ± 0.3 ms, P = 0.03; Ih fastτ/highA: 2.5 ± 0.2 ms, P < 0.01; n = 4, paired t test; Fig. 4A). Action potential delay in LSO neurons was not consistently altered by changing Ih properties (Fig. 4A).
We did not find action potentials to be delayed in the AVCN and the LSO due solely to alterations in Ih as previously reported for the MNTB (Leao et al. 2005a).
Rebound depolarizations
Hyperpolarizing current steps elicited rebound depolarizations in all three nuclei (Fig. 4B). Under control conditions, a −300 pA current step produced an average depolarization of 4.3 ± 0.9 mV in AVCN cells at the end of the step (Fig. 4B). Increasing the speed of Ih kinetics, with a V1/2 10 mV more depolarized, increased the post-step depolarization (Ih fastτ/lowA: 5.4 ± 2.2 mV, P = 0.05; Ih fastτ/highA: 7.0 ± 0.8 mV, P < 0.01; n = 4, paired t test; Fig. 4B). These differences were more dramatic in MNTB cells; altering both Ih amplitude and kinetics caused significant increases in rebound depolarization, and addition of Ih fastτ/highA (Fig. 4B) triggered post-step APs (control: 3.8 ± 0.5 mV; Ih slowτ/highA: 6.3 ± 0.3 mV, P = 0.01; Ih fastτ/lowA: 7.7 ± 0.7 mV, P ≤ 0.01; n = 4, paired t test; Fig. 4B). Interestingly, Ih properties did not seem to affect rebound amplitude in LSO cells except when Ih slowτ/lowA was added to the membrane (control: 6.2 ± 0.2 mV versus Ih slowτ/lowA: 6.9 ± 0.2 mV; P < 0.01, n = 5, paired t test; Fig. 4B). This larger depolarization was probably due to the decreased resting membrane potential, causing the relative increase after hyperpolarization potential. If consideration was taken as to whether the LSO cell was multiple or single firing, changes in Ih properties similarly affected resting membrane potential and AP characteristics in MF and SF LSO cells. However, rebound depolarizations in MF cells were able to trigger APs (see following subsections). The dynamic clamp data are summarized in Table 2.
Table 2.
Effect of Ih on resting membrane potential, action potentials and rebound depolarization in the AVCN, MNTB and LSO
| Vrest (mV) | AVCN | MNTB | LSO |
|---|---|---|---|
| Slowτ/lowA | −58.8 ± 1.7 | −65.3 ± 0.8 | −67.1 ± 0.2‡ |
| Slowτ/highA | −57.7 ± 1.7† | −61.7 ± 0.2† | −65.7 ± 0.4‡ |
| Fastτ/lowA | −56.1 ± 1.7‡ | −63.5 ± 0.6‡ | −64.6 ± 0.5‡ |
| Fastτ/highA | −53.7 ± 1.4‡ | −61.4 ± 0.5‡ | −61.3 ± 0.8 |
| AP amplitude (mV) | |||
| Slowτ/lowA | 62.8 ± 5.3 | 61.5 ± 4.1 | 99.8 ± 4.2† |
| Slowτ/highA | 62.5 ± 5.5 | 60.3 ± 4.0† | 96.5 ± 3.4† |
| Fastτ/lowA | 58.7 ± 5.5† | 57.8 ± 2.9 | 91.2 ± 3.7 |
| Fastτ/highA | 57.5 ± 5.9† | 56.3 ± 3.1† | 91.4 ± 4.4 |
| AP half-width (ms) | |||
| Slowτ/lowA | 1.33 ± 0.04 | 1.05 ± 0.09 | 0.70 ± 0.06 |
| Slowτ/highA | 1.14 ± 0.02‡ | 1.20 ± 0.12 | 0.67 ± 0.07 |
| Fastτ/lowA | 1.17 ± 0.02† | 1.21 ± 0.12 | 0.64 ± 0.07 |
| Fastτ/highA | 1.16 ± 0.01† | 1.13 ± 0.10 | 0.59 ± 0.04 |
| AP delay (ms) | |||
| Slowτ/lowA | 5.5 ± 0.4 | 3.1 ± 0.1 | 3.1 ± 0.3 |
| Slowτ/highA | 4.6 ± 0.3 | 2.7 ± 0.2† | 3.3 ± 0.5 |
| Fastτ/lowA | 4.6 ± 0.3 | 2.6 ± 0.3† | 3.0 ± 0.1 |
| Fastτ/highA | 4.5 ± 0.3 | 2.5 ± 0.2‡ | 3.4 ± 0.2 |
| Rebound dep (mV) | |||
| Slowτ/lowA | 4.3 ± 0.9 | 3.8 ± 0.5 | 6.9 ± 0.2‡ |
| Slowτ/highA | 5.4 ± 2.2 | 6.3 ± 0.3† | 7.0 ± 0.9 |
| Fastτ/lowA | 5.5 ± 1.3 | 7.7 ± 0.7‡ | 6.7 ± 0.2 |
| Fastτ/highA | 7.0 ± 0.8‡ | NA | 6.2 ± 0.2 |
Presented as mean ± S.E.M. Emboldened numbers represent control conditions. Significant difference from control condition
P < 0.05
P < 0.01, Student's paired t test. Rebound dep, rebound depolarisations. NA, not available.
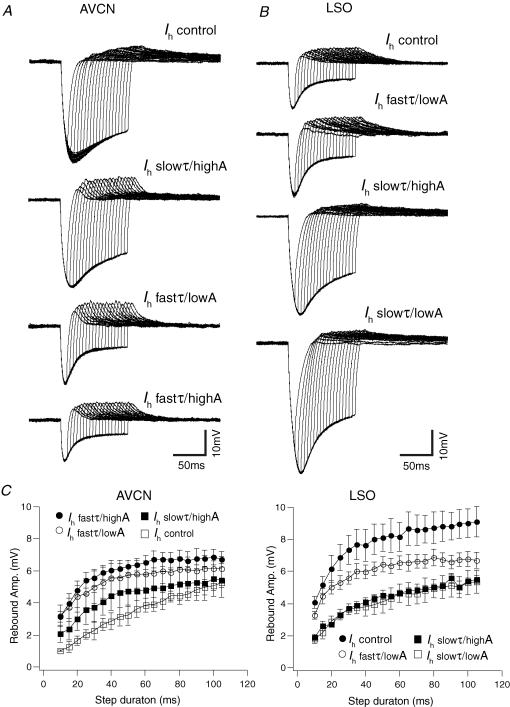
We did not observe any rebound APs in MNTB cells in a previous study (Leao et al. 2005), and Ih is unlikely to generate rebound APs in MNTB neurons under normal conditions. Therefore we further investigated the relationship between Ih properties and rebound depolarization in AVCN bushy cells and LSO principal neurons. In order to investigate the relationship between hyperpolarization duration, rebound depolarization amplitude, Ih properties and rebound firing, current steps (−300 pA) of various durations (10–110 ms, 5 ms steps; Koch & Grothe, 2003) were injected into AVCN and LSO cells as well as dynamic clamp Ih. AVCN neurons displayed a linear relationship between current-step duration and rebound depolarization under control conditions. In terms of depolarization amplitude, AVCN bushy cells did not show any difference between Ih control and slowτ/highA for any time step (Fig. 5A and C). Addition of a fast Ih, however, significantly increased rebound depolarization following steps from 45 to 55 ms for Ih fastτ/lowA (n = 4, P = 0.04 for all points) and after steps from 10 to 70 ms for Ih fastτ/highA (n = 4, P < 0.01 for all points). Also, under control conditions, the relationship between hyperpolarization step duration and depolarization potential could be well fitted with a linear function (e.g. y = ax+ b) while logarithmic functions (e.g. y = a log(x) + b) were more suited to fit this relationship if Ih amplitude was increased or its kinetics were changed (the goodness of fit was assessed by the magnitude of the residuals; data not shown).
Figure 5. Rebound depolarization shows different dependency on Ih in AVCN and LSO cells.
A, example of an AVCN bushy cell response to −300 pA hyperpolarizing current steps of various durations (10–105 ms, 5 ms increments) after different Ih properties had been applied by dynamic clamp to the same neuron. B, example of a SF LSO neuron voltage response to hyperpolarizing current steps (same as in A) with different Ih properties. C, summary of current step (−300 pA) duration versus rebound depolarization amplitude relationships for AVCN bushy cells and LSO principal neurons (data presented as mean ± s.e.m.) after different Ih properties had been applied by dynamic clamp.
Different Ih properties caused greater changes in the dependency of rebound depolarization on the hyperpolarizing current step duration in SF LSO cells (Fig. 5B and C). In general, rebound depolarizations were larger under control conditions (P < 0.05, when compared with Ih slowτ/lowA and Ih slowτ/highA for all steps, n = 5) except when compared with Ih fastτ /lowA (Fig. 5B and C). In contrast to AVCN neurons, all the hyperpolarizing step duration versus rebound amplitude relationships were better fitted with a logarithmic function. These results show that Ih fastτ/highA produces the largest rebound depolarization in AVCN cells and fastτ/lowA produce the largest depolarizations in LSO neurons, compared with control conditions.
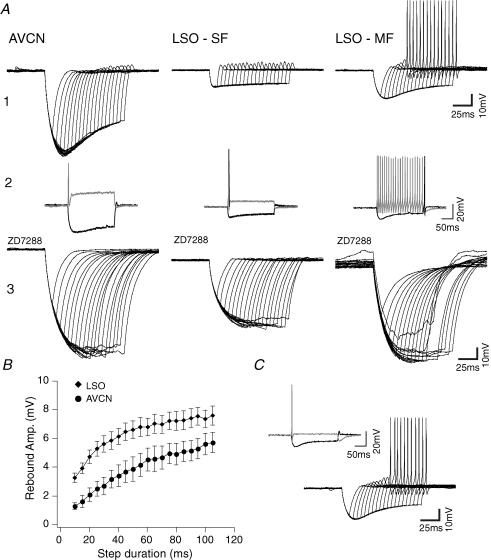
Rebound APs in response to hyperpolarizing current steps were observed in 29% of AVCN cells (n = 21) and in 35% of LSO cells (n = 20). Most of the LSO cells that fired rebound APs were MF cells (6 of 7 cells versus 1 of 13 SF cells). Rebound APs were eliminated by the addition of ZD7288 (Fig. 6A). Hyperpolarization durations necessary for rebound AP firing were significantly longer in AVCN cells when compared with LSO cells (58 ± 15 ms versus 26 ± 10 ms, P = 0.04, n = 10). AVCN bushy cells were invariably SF therefore the amount of ILT was measured by applying depolarizing voltage steps. After a voltage step to −40 mV there was a significant difference (P < 0.05) in outward current between AVCN bushy cells that fired rebound action potentials (235 ± 16 pA, n = 21) compared with AVCN bushy cells that did not fire rebound action potentials (340 ± 33 pA, n = 21). Non-rebound spiking AVCN bushy cells also showed smaller rebound depolarizations when compared with non-rebound spiking LSO cells (for 55 ms long hyperpolarization: 4 ± 1 mV for AVCN cells versus 7 ± 1 mV for LSO cells, P = 0.01, n = 22; Fig. 6B). We also artificially injected ILT using a dynamic clamp in four MF LSO cells in order to assess the effect of these currents on rebound spiking. Dynamic clamp K+ currents caused MF LSO cells to fire single spikes in response to depolarizing current steps and abolished rebound APs after −250 pA current steps (Fig. 6C, inset). However, these neurons still fired rebound APs after −300 pA current steps, but the hyperpolarization duration necessary for AP firing increased significantly (from 28 ± 18 to 62 ± 17 ms, P = 0.04, n = 4; Fig. 6C). We found that rebound depolarization magnitude depends jointly on Ih and ILT, as these two currents appear to balance each other (Ih promoting rebound depolarization after hyperpolarizations and ILT impeding rebound depolarizations to cause AP firing).
Figure 6. Rebound AP is dependent on Ih and low-threshold K+ current.
A1, example traces from an AVCN bushy cell, a LSO single-firing (SF) principal neuron and a LSO multiple-firing (MF) principal neuron after hyperpolarizing current injections of various durations (−300 pA, 10–105 ms, 5 ms increments). A2, AVCN, LSO SF and LSO MF membrane voltage response to a 200 pA and −300 pA current injection. A3, membrane voltage to different duration hyperpolarizing currents (as shown in A1) after addition of ZD7288. B, comparison of depolarization amplitudes after −300 pA current steps of different durations between non-rebound spiking AVCN and LSO cells (data presented as means ± s.e.m.). C, LSO MF cell (same as in A) response to the current protocol used in A after a low-threshold K+ current was artificially injected using dynamic clamp. Inset, LSO MF neuron (A2) voltage response to a 200 pA and −300 pA current injection after the addition of a simulated low-threshold K+ current.
Discussion
Our results show that the expression of the hyperpolarization-activated cation current, Ih, differs significantly between auditory brainstem nuclei. The magnitude of Ih is greater in LSO principal cells than AVCN bushy cells, which in turn exhibit an Ih significantly larger than in MNTB principal cells. The activation curve for Ih shows that the AVCN and MNTB cells have similar half-activation voltage, that is about −10 mV shifted towards hyperpolarized potentials compared with LSO principal cells. The activation kinetics of the LSO principal neurons were also significantly different to AVCN and the MNTB neurons. The activation kinetics of AVCN bushy cells were not significantly different to MNTB cells.
AVCN, MNTB and LSO cells express different combinations of HCN subunits
Neurons in the AVCN and SOC express more than one HCN channel subtype in combination. This HCN subunit composition, examined in the AVCN and the SOC using immunohistochemistry, agrees with our electrophysiological data measured in these nuclei. The slowly activating Ih in AVCN bushy cells is likely to be due to the expression of HCN4 channels, known to have slow kinetics. AVCN cells were also intensely labelled by anti-HCN1 antibodies, suggesting that HCN1 and HCN4 together account for the substantial Ih expressed by these cells. The observed variability in both amplitude and kinetics of Ih between AVCN neurons may therefore be due to differences in the proportion of HCN1 and HCN4 channels expressed in different neurons. MNTB cells appear to mostly express the slowly activating HCN2 and HCN4 channels, which may be reflected by the wide sag in response to negative current injections (Fig. 3). LSO cells strongly express HCN1 channels, in accordance with the fast activating Ih seen in these cells. This is in agreement with studies in rat displaying a strong labelling of HCN1 channels in LSO and AVCN and weak HCN1 staining in the MNTB, which instead had the strongest HCN2 staining of these three nuclei (Koch et al. 2004). Despite the fact that HCN1 is strongly expressed in both AVCN and LSO, Ih is substantially larger and faster in the latter. These differences might be related to heteromization of HCN channels. It has been shown elsewhere that Ih channel isoforms can exist as both homomers and heteromers (Moosmang et al. 1999; Santoro et al. 2000). Heteromers of the HCN1–HCN2 have been identified in mouse brain, indicating that heteromeric channels exist in vivo (Much et al. 2003). Some cells, such as basket and stellate cells of the cerebellum, exclusively express HCN1 but not HCN2, suggesting that HCN channels preferentially form homomeric complexes (Moosmang et al. 1999). Therefore, it is possible that HCN1 homomers are more ‘common’ in LSO neurons than AVCN bushy cells. Heteromeric HCN1–HCN4 channels have been studied in the rabbit heart, where neither HCN1 channel nor HCN4 channel activation could account for the native current seen in sino-atrial node cells alone, but a heteromeric expression of the two fitted activation kinetics and cAMP sensitivity (Altomare et al. 2003). This expression pattern might be applicable to AVCN bushy cells, accounting for an Ih magnitude that is between that of the largely HCN1-expressing LSO cells and the HCN4- and HCN2-expressing MNTB principal cells. This gradient of Ih magnitude was retained after heating the recording chamber to physiological temperature.
Ih kinetics are sensitive to temperature, with significantly decreased activation time constants for all cell types at physiological temperature compared with room temperature. Surprisingly, MNTB cells displayed faster Ih activation than the AVCN bushy cells in response to a −100 mV voltage step at 33–37°C. This could imply that the HCN4 channel subtype is more sensitive to temperature than the HCN1 channel, since the AVCN displays strong expression of both channel types. The activation constant for LSO cells was about twice as fast at physiological temperatures, compared with MNTB cells which displayed kinetics close to four times as fast.
In addition to different kinetics, there may also be differences in the modulation of Ih. Ih can be regulated by cAMP, which in its turn can be modified by neurotransmitters such as dopamine and adrenalin (Shepherd, 1994). However, this modulation is stronger in HCN2 and -4 subunits than for the HCN1 subunit. Our results demonstrate that HCN1 channels are the major carriers of Ih in LSO cells but not in the MNTB and AVCN, and therefore, catecholaminergic inputs could tonically alter the resting characteristics of MNTB (see Banks et al. 1993) and AVCN neurons but have little effect on LSO cells. Conversely, Ih in LSO neurons can be more ‘easily’ modulated by neural activity (e.g. APs and IPSPs) than Ih in AVCN and MNTB, as the kinetics of HCN1 are faster than the other subunits. This means that short bursts of APs are more likely to deactivate (and for IPSPs bursts, activate) Ih in the LSO than in the MNTB and AVCN.
Previous studies have shown that altered expression of single HCN channel subunits may have significant functional consequences. For example, knock-out of HCN1 in CA1 pyramidal neurons enhances performance of learning and memory tasks in mice (Nolan et al. 2004). It was proposed by Santoro & Baram (2003) that neuronal networks may regulate activity by up-regulating either the slow activating channels or the fast acting HCN1 channel. Slow kinetics of Ih has previously been suggested as a mechanism underlying neuronal tuning for sound duration (Casseday, 1994; Hooper, 2002). A recent study by Proenza & Yellen (2006) also focuses on distinct population of voltage-independent HCN channels. These channels could contribute to the instantaneous currents seen in current traces in response to negative voltage steps, which can accelerate both depolarization and repolarization.
Ih relationship to membrane potential and excitability
In this study we used a dynamic clamp to assess the effect of different types of Ih on cell excitability, by altering Ih amplitudes and kinetics. The results reveal effects on the resting membrane potential, with the membrane potential of AVCN bushy cells and LSO principal neurons more affected by changes in Ih compared with MNTB neurons. This difference suggests that in MNTB cells, other currents dominate around resting potential (e.g. twin-pore K+ channel currents) or that a strong ILT prevents changes in Ih from affecting membrane potential. The observation of more positive resting potentials in AVCN neurons, compared with MNTB cells, could indicate that ILT is less active in these cells, thus allowing changes in Ih to exert a greater influence on membrane potential. In LSO neurons, the strong hyperpolarization seen in resting conditions after Ih was decreased shows that Ih is a strong modulator of resting potential in these cells.
In vivo recordings have shown that blocking Ih leads to reduced excitability in the SOC (Shaikh & Finlayson, 2003). This effect is probably due to hyperpolarization of the resting membrane potential. Since the changes in membrane potential were greatest in LSO cells, this might indicate that Ih is more tonically active during rest in LSO neurons than in MNTB and AVCN neurons. This is also supported by the greater increase in input resistance after blocking Ih in LSO cells when compared with both MNTB and AVCN cells.
Ih and rebound depolarization
Another important aspect of this study was the assessment of the role of Ih in the generation of rebound over-threshold depolarizations. Activation of Ih by hyperpolarization is known to cause rebound APs in a variety of cells, including auditory neurons (Koch & Grothe, 2003; Ma et al. 2003). However, other ionic currents associated with Ih can prevent rebound APs (Ma et al. 2003). Here, we studied the effect of different Ih kinetics and amplitude on rebound firing in AVCN and LSO neurons, but not in the MNTB (because our previous study showed that it is unlikely that MNTB principal cells would fire rebound APs after prolonged activity; Leao et al. 2005). For both the AVCN and LSO, changing Ih characteristics did not cause rebound spikes in a previously non-rebound spiking neuron. Increasing amplitude or accelerating Ih kinetics altered the time dependency of hyperpolarization on rebound depolarization amplitudes in AVCN cells (from linear to logarithmic). This effect was probably due to larger rebound amplitudes activating low-threshold voltage-dependent K+ channels. Overall, rebound depolarizations were larger in LSO neurons when compared with AVCN cells, and more LSO neurons fired rebound APs compared with AVCN bushy cells. The strong correlation between small ILT and rebound spiking in AVCN cells suggested that these currents prevent rebound depolarizations from reaching AP threshold. The same hypothesis is applicable to LSO cells, where multiple firing LSO cells, exhibiting less ILT (Barnes-Davies et al. 2004), fired rebound APs with a greater frequency than single firing cells. By simulating ILT, in LSO cells using the dynamic clamp, rebound APs could be strongly inhibited, supporting this theory. However, these dynamic clamp experiments failed to abolish rebound APs after application of long hyperpolarization protocols, probably because the activation of ‘real’ ILT in LSO cells might be faster than the simulated current used in this work. We conclude that the rebound depolarization or rebound AP is modulated by the balance between Ih and ILT activity.
In summary, this study highlights significant differences in Ih characteristics between different auditory brainstem nuclei, with each cell type expressing a distinct repertoire of Ih and HCN channel subtypes. Our results show Ih to be a key modulator of cell excitability that can be crucial for synaptic integration and coincidence detection.
References
- Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D. Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol. 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. J Neurophysiol. 2000;84:806–817. doi: 10.1152/jn.2000.84.2.806. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol. 1993;70:1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. doi: 10.1111/j.0953-816x.2003.03133.x. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Enrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 1994;264:847–850. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- Chen C. Hyperpolarization-activated current (Ih) in primary auditory neurons. Hear Res. 1997;110:179–190. doi: 10.1016/s0378-5955(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Cuttle MF, Rusznak Z, Wong AY, Owens S, Forsythe ID. Modulation of a presynaptic hyperpolarization-activated cationic current (Ih) at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol. 2001;534:733–744. doi: 10.1111/j.1469-7793.2001.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL, Buchman E, Hobbs KH. A computational role for slow conductances: single-neuron models that measure duration. Nat Neurosci. 2002;5:552–556. doi: 10.1038/nn0602-838. [DOI] [PubMed] [Google Scholar]
- Koch U, Braun M, Kapfer C, Grothe B. Distribution of HCN1 and HCN2 in rat auditory brainstem nuclei. Eur J Neurosci. 2004;20:79–91. doi: 10.1111/j.0953-816X.2004.03456.x. [DOI] [PubMed] [Google Scholar]
- Koch U, Grothe B. Hyperpolarization-activated current (Ih) in the inferior colliculus: distribution and contribution to temporal processing. J Neurophysiol. 2003;90:3679–3687. doi: 10.1152/jn.00375.2003. [DOI] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe REW, Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol. 2006;571:563–578. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Svahn K, Berntson A, Walmsley B. Hyperpolarization-activated (I) currents in auditory brainstem neurons of normal and congenitally deaf mice. Eur J Neurosci. 2005;22:147–157. doi: 10.1111/j.1460-9568.2005.04185.x. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ma YP, Cui J, Hu HJ, Pan ZH. Mammalian retinal bipolar cells express inwardly rectifying K+ currents (IKir) with a different distribution than that of Ih. J. Neurophysiol. 2003;90:3479–3489. doi: 10.1152/jn.00426.2003. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Biel M, Hofmann F, Ludwig A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol Chem. 1999;380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Proenza C, Yellen G. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J General Physiol. 2006;127:183–190. doi: 10.1085/jgp.200509389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Baram TZ. The multiple personalities of h-channels. Trends Neurosci. 2003;26:550–554. doi: 10.1016/j.tins.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Grant SG, Bartsch D, Kandel ER. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Finlayson PG. Hyperpolarization-activated (Ih) conductances affect brainstem auditory neuron excitability. Hear Res. 2003;183:126–136. doi: 10.1016/s0378-5955(03)00224-7. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Neurobiology. New York: Oxford University Press; 1994. [Google Scholar]
- Yamada R, Kuba H, Ishii TM, Ohmori H. Hyperpolarization-activated cyclic nucleotide-gated cation channels regulate auditory coincidence detection in nucleus laminaris of the chick. J Neurosci. 2005;25:8867–8877. doi: 10.1523/JNEUROSCI.2541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]