Abstract
Background
Bordetella pertussis, the causative agent of whooping cough, is a highly clonal pathogen of the respiratory tract. Its lack of genetic diversity, relative to many bacterial pathogens, could limit its ability to adapt to a hostile and changing host environment. This limitation might be overcome by phase variation, as observed for other mucosal pathogens. One of the most common mechanisms of phase variation is reversible expansion or contraction of homopolymeric tracts (HPTs).
Results
The genomes of B. pertussis and the two closely related species, B. bronchiseptica and B. parapertussis, were screened for homopolymeric tracts longer than expected on the basis of chance, given their nucleotide compositions. Sixty-nine such HPTs were found in total among the three genomes, 74% of which were polymorphic among the three species. Nine HPTs were genotyped in a collection of 90 geographically and temporally diverse B. pertussis strains using the polymerase chain reaction/ligase detection reaction (PCR/LDR) assay. Six HPTs were polymorphic in this collection of B. pertussis strains. Of note, one of these polymorphic HPTs was found in the fimX promoter, where a single base insertion variant was present in seven strains, all of which were isolated prior to introduction of the pertussis vaccine. Transcript abundance of fimX was found to be 3.8-fold lower in strains carrying the longer allele. HPTs in three other genes, tcfA, bapC, and BP3651, varied widely in composition across the strain collection and displayed allelic polymorphism within single cultures.
Conclusion
Allelic polymorphism at homopolymeric tracts is common within the B. pertussis genome. Phase variability may be an important mechanism in B. pertussis for evasion of the immune system and adaptation to different niches in the human host. High sensitivity and specificity make the PCR/LDR assay a powerful tool for investigating allelic variation at HPTs. Using this method, allelic diversity and phase variation were demonstrated at several B. pertussis loci.
Background
Bordetella pertussis causes whooping cough, a highly communicable disease that killed roughly 279,000 people and infected 17.6 million people globally in a recent typical year [1]. B. pertussis and the closely related human- and sheep-adapted species, B. parapertussis, have diverged independently by genome decay from a putative common ancestor that they share with B. bronchiseptica, which has a broader host range, and unlike the other two species, causes chronic infections [2-4]. Re-emergence of pertussis in certain vaccinated populations [5,6] suggests that B. pertussis may be adapting to vaccine-induced host immunity [5]. In support of this hypothesis, shifts in the allelic frequencies of genes encoding at least three vaccine components (pertussis toxin, pertactin, and fimbriae) have been documented in B. pertussis from Finland, Sweden, France and the Netherlands [5,7-9]. However, multilocus enzyme electrophoresis (MLEE) [10], comparative genome hybridization (CGH) [11] and multilocus sequence typing (MLST) of seven housekeeping genes [4] have established that B. pertussis is highly clonal with nearly invariant genome content. The apparent scarcity of variation in the B. pertussis genome is unusual among bacterial pathogens, in which extensive genomic plasticity is thought to contribute to host immune evasion [12].
Phase variation is one means by which B. pertussis might adapt to host immune surveillance and vaccine-induced immune responses without loss or acquisition of genomic fragments [13]. In a number of pathogenic bacteria, phase variation influences expression of virulence phenotypes and creates phenotypic diversity in clonal populations (reviewed in [14,15]). Phase variation can also influence the affinity of pathogens for different host anatomical niches. For example, variable expression of colony opacity (Opa) proteins changes the tropism of Neisseria for human epithelium, endothelium, and phagocytic cells [16].
The genetic mechanism of phase variation in many cases is expansion and contraction of nucleotide repeat sequences (reviewed in [17,18]). Homopolymeric nucleotide tracts (HPTs) frequently grow or shrink, with longer HPTs more prone to slippage. By searching bacterial genome sequences for unusually long HPTs, putative phase-variable genes have been identified, and in some cases, validated [19-22].
B. pertussis undergoes variation between virulent and avirulent phases [23]. The molecular mechanism of this phenotypic switch is expansion and contraction of an HPT in bvgS [24], which encodes a key regulator of virulence gene expression (reviewed in [25]). In addition, phase-variable expression of two fimbrial major subunit genes, fim2 and fim3, has been attributed to variations in HPTs located within the promoter regions of these genes that lead to altered transcript abundance [26,27]. In this study, we sought to identify additional B. pertussis candidate phase-variable genes, particularly those with potential roles in pathogenesis. Furthermore, we sought to test whether these genes were polymorphic in the B. pertussis population and, specifically, whether they were phase-variable over the short time period that characterizes an infectious cycle.
In order to measure HPT tract length in a rapid, sensitive, and accurate manner in a large collection of B. pertussis strains, the polymerase chain reaction/ligase detection reaction (PCR/LDR) was employed. This assay has been used to study genomic repeat instability in human tumors [28] and has been applied to the identification of rifampin-resistant Mycobacterium tuberculosis strains [29]. The ability of PCR/LDR to identify rare genotypic variants in a population, and its high fidelity, make this method well suited for detection of HPT-associated phase variation events. Using this method, HPT length polymorphisms were demonstrated at six out of nine putative phase-variable loci, among a collection of 90 B. pertussis isolates. Two of these loci, both encoding Bvg-activated surface proteins, were found to exhibit rapid and reversible mutations. Phase variation in B. pertussis is more common than previously recognized and may be a significant source of phenotypic diversity in this pathogen.
Results
Validation of PCR/LDR for detection of HPT alleles in bacterial genomes
Variation between virulent and avirulent phenotypic phases in B. pertussis has been attributed to a polymorphism within an HPT located in the coding region of the virulence regulatory sensor kinase gene, bvgS [24]. Virulent strains (e.g., BP370) have 6 cytosines (C6) in this HPT and produce full-length BvgS. The HPT of avirulent derivative strains (e.g., BP369) has 7 cytosines (C7), leading to a translational frameshift that truncates BvgS, rendering it non-functional [24].
In order to test the utility of PCR/LDR for detecting HPT polymorphisms, oligonucleotides were designed for discrimination of the C6 and C7 alleles of bvgS (Figure 1A). A bvgS fragment containing the HPT was amplified by PCR using a high-fidelity, thermostable DNA polymerase (Pfu Ultra). Each LDR reaction contained the amplified template DNA, thermostable Taq DNA ligase, a 5-carboxyfluorescein (FAM)-labeled "common" oligonucleotide, and one or more unlabeled "discriminating" oligonucleotides that differ in length from each other by at least three nucleotides so as to enhance electrophoretic separation of the ligation products. The common oligonucleotide was designed to be complementary to 3 bases of the HPT plus 15 bases of downstream sequence. Both the C6 and C7 discriminating oligonucleotides were complementary to 14 bases of the upstream flanking sequence, but they differed in the number of repeated bases at the 3' end: the C6 discriminating oligonucleotide had three repeated bases, and the C7 discriminating oligonucleotide had four. When the sum of the repeated bases at the 3' end of the discriminating oligonucleotide and the 5' end of the common oligonucleotide equals the number of repeated bases in the template HPT, the alignment of the oligos on the template favors the formation of a single-stranded ligation product with diagnostic size (Figure 1A).
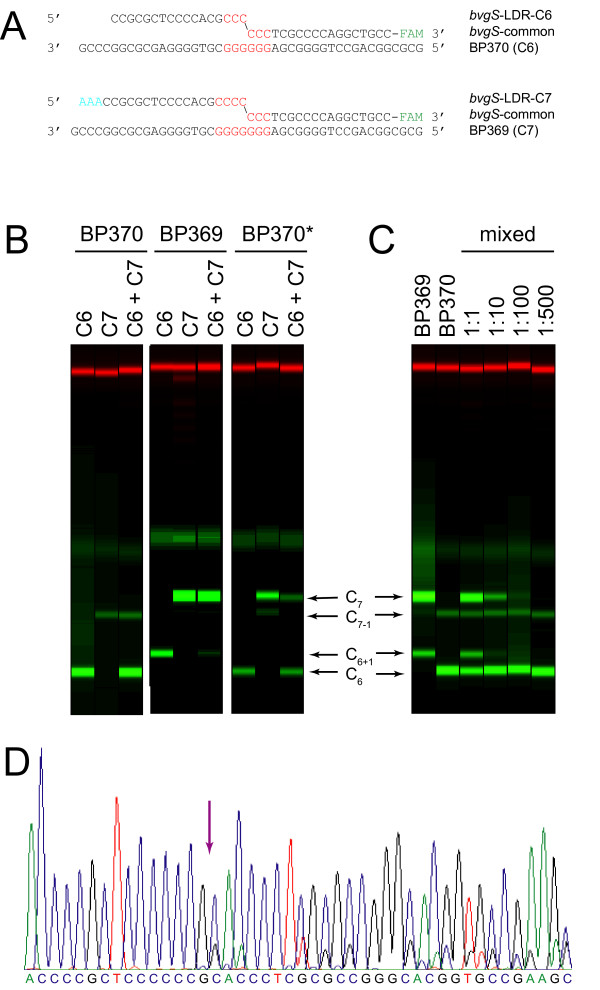
Figure 1.
Detection by PCR/LDR of HPT alleles in bvgS. (A) Schematic of BP370 and BP369 genomic DNA templates aligned with LDR oligonucleotides. Purple text denotes the HPT; green star indicates the FAM fluorophore; cyan text shows non-hybridizing bases used for size discrimination of oligos. HPT length is six cytosines (C6) in BP370, and seven cytosines (C7) in BP369. Covalent bonds (diagonal lines) are catalyzed by DNA ligase only if the discriminating and common oligonucleotides are immediately adjacent when hybridized to the template. (B) Raw capillary electrophoresis data for ligation products (green) and molecular weight standards (red) displayed as if an electrophoretic gel image. Source of template genomic DNA is indicated across the top of the panel. BP370* is a culture derived from BP370 that contains a minority population of C7 phase variants. All reactions contained common oligonucleotide; discriminating oligonucleotides are indicated above each lane (C6 and C7 indicate uniplex reactions, C6 + C7 indicates multiplex). Ligation products (arrows) are labeled as follows. C7 and C6 indicate the products formed by ligation of the common oligonucleotide to the C7 and C6 discriminating oligonucleotides, respectively. C6+1 denotes the product formed by ligation of the common oligonucleotide to a synthesis artifact in the C6 discriminating oligonucleotide preparation that has one extra 3' C. Similarly, C7-1 denotes the product formed by ligation of the common oligonucleotide to a synthesis artifact in the C7 discriminating oligonucleotide preparation that has one fewer 3' C. This notation is used throughout the figures of this manuscript. (C) Raw capillary electrophoresis displayed as in panel B. All lanes are multiplex reactions using C6 and C7 discriminating oligos with the genomic DNA template indicated above each lane. Lanes 3–6 show the ratio of PCR amplified bvgS from BP369 to bvgS from BP370. Ligation products are labeled as in panel B. (D) Partial trace of directly sequenced bvgS PCR product from BP370*. Purple arrow indicates the point in the sequence trace after which peak shadowing, indicative of a mixture of HPT alleles, can be observed.
As expected, PCR/LDR analysis of the virulent BP370 strain with the C6 discriminating oligonucleotide yielded an abundant ligation product (Figure 1B). However, PCR/LDR with the C7 discriminating oligonucleotide preparation also produced a ligation product, albeit more faint. This finding can be attributed to oligonucleotide synthesis errors (see capillary electrophoresis data in Additional file 1) that cause a fraction of the oligonucleotides (denoted "C7-1") in the HPLC-purified synthesis product to contain one fewer cytosine at the 3' end. These rare synthesis artifacts can, in the presence of a C6 template, form a detectable ligation product. This interpretation is further supported by the lower mass of the C7-1 ligation product compared to the C7 ligation product that would be formed by the full-length C7 oligonucleotide in the presence of a C7 template. Conversely, PCR/LDR of the avirulent parent, BP369, generated an abundant ligation product with the C7 discriminating oligonucleotide, as expected. However, as above, a faint ligation product produced with the C6 discriminating oligonucleotide preparation, with higher mass than expected for the C6 product, can be attributed to synthesis errors that result in rare oligonucleotides (denoted "C6+1") with one additional cytosine at the 3' end. Ligation products formed by synthesis artifacts could be easily distinguished from legitimate products by their characteristic electrophoretic mobility. Equivalent ligation products were obtained whether reactions contained one (uniplex) or both (multiplex) discriminating oligonucleotides (Figure 1B).
In order to examine the ability of PCR/LDR to detect infrequent HPT alleles in a mixed population of templates, bvgS PCR product from BP369 was mixed with bvgS PCR product from BP370 in a series of dilutions (Figure 1C). The C7 allele from BP369 could be detected in a multiplex reaction when present at 100-fold lower concentration than the C6 allele, but not at 500-fold lower concentration. This sensitivity is similar to that observed for PCR/LDR detection of single nucleotide HPT variants on synthetic templates [28]. Thus, this method can detect phase variants that comprise as little as 1% of the sample template.
To assess the sensitivity of PCR/LDR in identifying a mixture of alleles in a bacterial sample, genomic DNA was prepared from a culture of BP370*, a strain that was isolated during a single passage of BP370 on BG blood agar. As the result of spontaneous mutation, BP370* contains a mixture of virulent and avirulent phase variants, as determined by plating on BG blood agar. Multiplex PCR/LDR of this sample yielded ligation products with both the C6 and C7 discriminating oligonucleotides, verifying that the genomic DNA preparation from this strain contained a mixture of C6 and C7 bvgS alleles (Figure 1B). This result was further validated by DNA sequencing of the bvgS PCR product from the same DNA preparation (Figure 1D). Thus, cultures composed of mixed populations with distinct HPT alleles, such as those that arise during laboratory passage of B. pertussis, are readily identifiable by PCR/LDR.
The rate of repeat expansion and contraction due to DNA polymerase slippage rises with increasing repeat number [30]. Therefore, high-fidelity PCR amplification is critical to this allelic detection scheme, especially for long HPT tracts. In order to asses the fidelity of PCR/LDR, a region of bapC containing a G10 tract was amplified using either high-fidelity Pfu DNA polymerase or the relatively lower fidelity Taq DNA polymerase, then screened by LDR (as described below). After 30 cycles of PCR, LDR of the Pfu-amplified template showed no tract length variation, but LDR of the Taq-amplified template detected tracts of 9, 10, and 11 guanosines (Additional file 2). Subsequent rounds of PCR product dilution and re-amplification with Pfu polymerase did not yield any detectable HPT variants (contractions) until the third round (90 cycles of PCR); tract expansions were not observed even after 120 cycles. Therefore, in a standard PCR/LDR experiment (30 cycles of PCR), there is negligible variation in HPTs 10 bases or shorter due to polymerase replication error when Pfu DNA polymerase is utilized. These results indicate that tract length heterogeneity detected by PCR/LDR as performed in this study accurately reflects HPT length heterogeneity in vivo.
Identification of putative phase-variable genes in Bordetella genomes
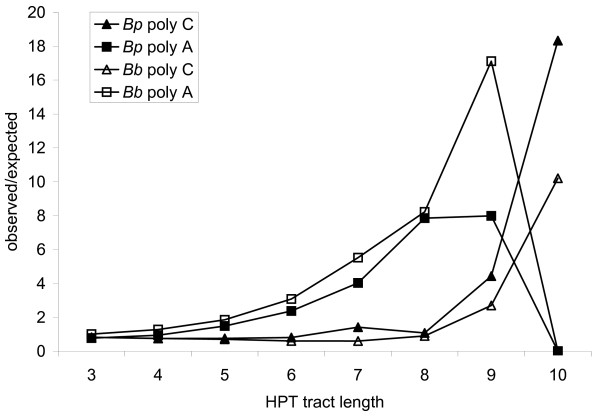
To identify putative phase-variable genes in B. pertussis, the genome sequence of the Tohama I strain [3] was screened for HPTs. Statistical analysis of sequence composition with a second order Markov model indicated that C/G HPTs more than 8 bases long, and A/T HPTs more than 5 bases long were observed more frequently than would be expected by chance in the B. pertussis and B. bronchiseptica genome sequences (Figure 2). To be conservative, the threshold for calling A/T HPTs was raised to greater than 7, due to the relative stability of shorter repeats in experimental systems [31]. Although shorter HPTs are known to be phase-variable in B. pertussis (e.g., C6 in bvgS), only genes associated with HPTs exceeding these conservative thresholds were classified as putative phase-variable genes in this computational screen. Because only a single B. pertussis genome sequence is available, it was not possible to search for polymorphic HPTs between strains of this species. However, because B. pertussis and B. parapertussis recently evolved from B. bronchiseptica [2-4], orthologous sequences of these three species are highly conserved. Of particular relevance to this study, single nucleotide indels between the B. pertussis and B. bronchiseptica genomes are found at fewer than 1 in 10,000 bases. Therefore, any HPTs that are polymorphic among these three species were strong candidates for phase-variable loci. In accordance with this logic, the B. parapertussis and B. bronchiseptica genomes [3] were also screened for HPTs, and for each tract, the orthologous locus was examined in all three species. This screen resulted in the identification of 69 HPT loci among the three species (Table 1; Additional files 3, 4, and 5). Because sequence composition differs in mobile genetic elements, HPTs in prophage or insertion elements were excluded from further analysis.
Figure 2.
Observed/expected plots of different length HPTs in the B. pertussis and B. bronchiseptica genomes. Expected frequencies of A and C HPTs in the B. pertussis Tohama I (Bp) and B. bronchiseptica RB50 (Bb) genomes were calculated for a range of tract lengths using a second order Markov model. For each tract length, observed/expected values were determined by dividing the actual number of HPTs greater than or equal to that tract length by the expected number. The observed/expected value for A HPTs drops to zero at tract length of 10 because neither genome harbors an A HPT with more than 9 bases.
Table 1.
Significant HPTs in B. pertussis, B. bronchiseptica, and B. Parapertussis
| B. pertussis Tohama I | B. bronchiseptica RB50 | B. parapertussis 12822 | ||||||||||
| HPTa | ORFb | Allelec | ORF | Allele | ORF | Allele | Gene | Base | Poly-morphicd | Loce | Promoter overlapf | Functional categoryg |
| 11 | BP2991 | 7 | BB4384 | 9 | BPP3911 | 9 | A/T | y | I | L | Biosynthesis of cofactors, carriers | |
| 12 | BP3682 | 8 | BB0097 | 8 | BPP0098 | 8 | A/T | n | I | M | Hypothetical protein | |
| 13 | BB0618, BB0619 | 8 | BPP0612, BPP0613 | 7 | A/T | y | I | H | Global regulatory functions | |||
| 14 | BB0622 | 8 | BPP0616 | 8 | A/T | n | I | M | Conserved hypothetical | |||
| 15 | BP3151 | 9 | BB0875 | 8 | BPP0790 | 8 | A/T | y | I | L | Cell envelope | |
| 16 | BB0999 | 8 | BPP0906 | 8 | A/T | n | I | L | Transport/binding proteins | |||
| 17 | BP0880 | 7 | BB1564 | 8 | BPP2167 | 7 | A/T | y | I | L | Periplasmic/exported/lipoproteins | |
| 18 | BP2792 | 8 | BB1918 | 8 | BPP2471 | 8 | A/T | n | I | H | Hypothetical protein | |
| 19 | BP2908 | 8 | BB1935 | 8 | BPP2488 | 8 | aroG | A/T | n | I | L | Amino acid biosynthesis |
| 20 | BP2735 | 8 | BB2036 | 8 | BPP2594 | 8 | A/T | n | I | H | Conserved hypothetical | |
| 21 | BP2539 | 8 | BB2083 | 8 | BPP2640 | 8 | A/T | n | I | L | Cell envelope | |
| 23 | BP1487 | 8 | BB2136 | 8 | BPP1948 | 8 | smoMh | A/T | n | I | M | Transport/binding proteins |
| 24 | BP1419 | 8 | BB2605 | 8 | BPP1527 | 8 | rpsB | A/T | n | I | M | Ribosome constituents |
| 25 | BP1245 | 9 | BB3248 | 8 | BPP1860 | 11 | A/T | y | I | M | Conserved hypothetical | |
| 26 | BP1231 | 8 | BB3262 | 8 | BPP1846 | 8 | A/T | n | I | H | Cell envelope | |
| 27 | BP2399 | 8 | BB3715 | 8 | BPP3264 | 7 | A/T | y | I | L | Global regulatory functions | |
| 28 | BP2425 | 8 | BB3740 | 8 | BPP3289 | 9 | A/T | y | I | M | Periplasmic/exported/lipoproteins | |
| 29 | BP0858 | 9 | BB3824 | 8 | BPP3373 | 9 | A/T | y | I | H | Hypothetical protein | |
| 30 | BP0856 | 8 | BB3826 | 8 | BPP3376 | 10 | bfrD | A/T | y | I | L | Adaptation |
| 31 | BP3399 | 8 | BB3987 | 8 | BPP3552 | 8 | A/T | n | I | M | Conserved hypothetical | |
| 32 | BB4499 | 8 | BPP4026 | 8 | A/T | y | I | H | Conserved hypothetical | |||
| 33 | BP0347 | 8 | BB4655 | 8 | BPP4185 | 8 | bhuR | A/T | n | I | K | Adaptation |
| 34 | BP3870 | 8 | BB5012 | 8 | BPP4424 | 8 | A/T | n | I | M | Hypothetical protein | |
| 41 | BP2506, BP2507 | 9 | BB3941, BB3942 | 9 | BPP3494 | 9 | A/T | n | I | N | Macromolecule synthesis, modification | |
| 43 | BP2863 | 9 | BB1178 | 9 | BPP0966 | 9 | A/T | n | I | H | Conserved hypothetical | |
| 50 | BP0939 | 8 | A/T | y | I | L | Conserved hypothetical | |||||
| 51 | BP0964 | 8 | BB3455 | 9 | BPP3115 | 9 | A/T | y | I | L | Hypothetical protein | |
| 52 | BP0966 | 8 | BB3453 | 9 | BPP1655 | 9 | sbp | A/T | y | I | M | Transport/binding proteins |
| 56 | BP2936 | 8 | BB1280 | 8 | BPP1064 | 9 | A/T | y | I | M | Periplasmic/exported/lipoproteins | |
| 60 | BP0598 | 6 | BB0289 | 6 | BPP0286 | 8 | A/T | y | I | L | Conserved hypothetical | |
| 64 | BB1238 | 7 | BPP1024 | 8 | A/T | y | I | M | Energy metabolism, carbon | |||
| 68 | BP2084 | 7 | BB3340 | 7 | BPP1768 | 8 | A/T | y | I | H | Conserved hypothetical | |
| 69 | BP1811 | 6 | BB3216 | 6 | BPP1892 | 8 | kdgT | A/T | y | I | M | Transport/binding proteins |
| 70 | BP1781 | 6 | BB2260 | 7 | BPP2012 | 8 | A/T | y | C | Conserved hypothetical | ||
| 73 | BPP2251 | 8 | bapA | A/T | y | I | L | Cell envelope | ||||
| 76 | BP1893, BP1894i | A2CA6 | BB2980, BB2979i | A2CA6 | BPP3014, BPP3013i | 9 | A/T | y | I | H | Cons. hypo.; Global reg. functions | |
| 79 | BP3852 | nrj | BB4994 | 7 | BPP4406 | 8 | katA | A/T | y | I | L | Protection responses |
| 83 | BB3111, BB3112i | 8 | BPP1616, BPP1617i | 8 | A/T | n | I | L | Cell envelope; hypothetical protein | |||
| 84 | tRNA-Asn, tRNA-Thri | 8 | tRNA-Asn, tRNA-Thri | 8 | tRNA-Asn, tRNA-Thri | 8 | A/T | n | I | U | Macromolecule synthesis, modification | |
| 1 | BB0357 | 11 | BPP0354 | 8 | C/G | y | I | M | Periplasmic/exported/lipoproteins | |||
| 2 | BB1186 | 10 | BPP0974 | 7 | C/G | y | I | M | Periplasmic/exported/lipoproteins | |||
| 3 | BP1568 | 13 | BB1658 | 22 | BPP2262 | C6TC3 | fim3 | C/G | y | I | K | Cell envelope |
| 4 | BP2738 | 11 | BB2033 | 7 | BPP2591 | 7 | bapCh | C/G | y | C | Cell envelope | |
| 5 | BB3110 | 17 | BPP1618 | nr | C/G | y | C | Cell envelope | ||||
| 6 | BP1201 | 9 | BB3291 | 17 | tcfA | C/G | y | C | Cell envelope | |||
| 7 | BB3425 | 15 | BPP1683 | 10 | fimN | C/G | y | I | K | Cell envelope | ||
| 8 | BP2674 | 7 | BB3426 | 13 | BPP1682 | 9 | fimX | C/G | y | I | K | Cell envelope |
| 9 | BP1119 | 12 | BB3674 | 20 | BPP3222 | C9TC8 | fim2 | C/G | y | I | K | Cell envelope |
| 10 | BP3037, BP3038 | 11 | BB4210, BB4211 | 11 | BPP3764, BPP3765 | 9 | C/G | y | I | N | Hypothetical protein | |
| 22 | BP1487 | 9 | BB2136 | C5GC3 | BPP1948 | C5GC3 | smoMh | C/G | y | C | Transport/binding proteins | |
| 35 | BP0146 | 15 | BB4482 | 6 | BPP4009 | 6 | C/G | y | I | H | Hypothetical protein | |
| 36 | BP1766 | 10 | BB2245 | G6TG3 | BPP1997 | 8 | C/G | y | I | N | Central intermediary metabolism | |
| 37 | BP1879 | 10 | BB2993 | 3 | BPP3027 | 2 | fhaB | C/G | y | C | Cell envelope | |
| 39 | BP1961 | 11 | BB1784 | nr | BPP2333 | nr | C/G | y | I | H | Energy metabolism, carbon | |
| 40 | BP2483 | 10 | BB3920 | C2AC5 | BPP3471 | C2AC5 | kdpD | C/G | y | C | Global regulatory functions | |
| 42 | BP2595 | 12 | BB2360 | G3TG3 | BPP1295 | G3TG3 | C/G | y | C | Macromolecule degradation | ||
| 44 | BP2896 | 9 | BB1947 | 8 | BPP2500 | 7 | C/G | y | I | H | Hypothetical protein | |
| 45 | BP3224 | 9 | BB4011 | 6 | BPP3576 | nr | C/G | y | C | Energy metabolism, carbon | ||
| 46 | BP3474 | 13 | BB0944 | 9 | BPP0850 | 7 | C/G | y | I | M | Conserved hypothetical | |
| 59 | BP3624 | 7 | BB0040 | 7 | BPP0040 | 9 | C/G | y | I | H | Degradation of small molecules | |
| 61 | BP0520 | 6 | BB0444 | 5 | BPP0443 | 9 | C/G | y | I | L | Periplasmic/exported/lipoproteins | |
| 62 | BP0426 | nr | BB0472 | 7 | BPP0472 | 13 | cspA | C/G | y | I | L | Adaptation |
| 65 | BB1351 | 9 | BPP1135 | 9 | C/G | n | I | H | Global regulatory functions | |||
| 66 | BP1047 | 7 | BB1361 | 8 | BPP1145 | 10 | C/G | y | I | M | Periplasmic/exported/lipoproteins | |
| 67 | BP1160 | 8 | BB2684 | 8 | BPP1607 | 11 | C/G | y | C | Periplasmic/exported/lipoproteins | ||
| 71 | BP1793 | 2 | BB2270 | 18 | BPP2022 | G12TG2AG3 | C/G | y | I | L | Cell envelope | |
| 75 | BP1660 | G5CG2CG3 | BB2741 | 8 | BPP2745 | 12 | sphB2 | C/G | y | C | Cell envelope | |
| 78 | BB4881 | G4ACG2 | BPP4294 | 14 | C/G | y | I | H | Global regulatory functions | |||
| 82 | BP2738 | 14 | BB2033 | 35 | BPP2591 | C6TC5 | bapCh | C/G | y | C | Cell envelope | |
a HPT number is an arbitrarily assigned unique ID. Table is sorted by order in the B. bronchiseptica RB50 genome.
b Identifier of ORF that is either contains or is downstream of the HPT. Empty cell indicates that no orthologous sequence exists in that genome.
c Numbers are length of HPTs. Sequences are given when the tract contains a substitution relative to alleles in the other genomes.
d "y" denotes HPT length polymorphism among the three sequenced genomes.
e "I", located in intergenic region; "C", located in coding sequence
f Probability that the HPT overlaps a promoter coded as follows: H, high; M, moderate; L, low; N, not near a promoter (located in 3' region of convergently transcribed genes); K, known to overlap experimentally defined promoter; U, undetermined. Values were not assigned to coding HPTs. See Methods for definitions of classes.
g Functional categories from MultiFun classification system as assigned by Parkhill, et al. [3]
h Two separate HPTs are found in these genes.
i HPT is upstream of two features by a similar distance.
j "nr" indicates that no HPT is found in the orthologous region.
Among the three species, 51 (74%) of the HPT loci were found to be polymorphic, meaning that the orthologous locus is present in at least two genomes and HPT length differs between them (Table 1). As a result, some of the HPTs in one or two genomes have fewer bases than the selection threshold. Twelve of the HPTs were found in coding regions, with the potential to influence protein length or amino acid sequence. In order to identify tracts that could affect transcriptional regulation, intergenic HPTs were assigned a likelihood of overlapping a promoter, with those located between 21 and 80 nt upstream of the start codon of an ORF being the most likely candidates (Table 1; Additional files 3, 4, and 5). Nineteen HPTs are in this class, including those upstream of the four fimbrial major subunit genes where they have been shown to overlap the functional promoters [26,27]. Although it is more than 80 nt upstream of the start codon, the HPT upstream of bhuR was also considered very likely to influence transcription because it maps five bases upstream of the experimentally defined -35 box [32]. In most cases, due to orientation or relative proximity of flanking CDSs, intergenic HPTs could be associated with the regulatory region of a single gene. Intergenic HPTs located in the 3' region of two ORFs or more than 150 nt upstream of the closest ORF start codon are unlikely to be involved in regulation.
Sixteen HPTs were associated with genes encoding cell envelope proteins, and 8 were associated with genes encoding periplasmic or exported proteins, or lipoproteins, for a total of at least 24 genes whose putative products are possible targets for host cell interaction and immune surveillance. Polymorphisms among the three species were more frequent among G/C HPTs (29/30) than among A/T HPTs (21/39) (two-tailed p = 0.00007, Fisher's exact test), but this result could be due to the longer tract length of G/C HPTs analyzed here. Due to gene loss in B. pertussis, 13 of the Bordetella HPT loci identified here are not present in the Tohama I genome. Also, the genes linked to two HPT loci identified in the other species are present in Tohama I, but they are not associated with an orthologous HPT, and two other loci have stabilized HPTs in Tohama I. In summary, we assessed B. pertussis to have 36 HPTs that are potentially subject to phase variation, 10 by disrupting an open reading frame, and 26 with high or moderate likelihood of affecting transcriptional regulation. By similar logic, B. bronchiseptica and B. parapertussis were assessed to have 43 and 42 putative phase-variable HPTs, respectively.
Identification of B. pertussis phase-variable HPTs by PCR/LDR
In order to detect rare tract length alleles in the B. pertussis population, and identify variations that may be associated with specific time periods or geographical locations, a large collection of isolates (see below) was assayed for a selected group of HPTs. Six loci were chosen from the list of Bordetella HPTs for further investigation of allelic polymorphism (Table 1). Selection of these loci was biased toward HPTs in genes encoding predicted cell envelope proteins (four HPTs) and HPTs that were polymorphic among the three sequenced genomes (five HPTs). Three HPTs – upstream of fimX (BP2674), BP0880, and bhuR – are predicted to influence transcriptional regulation. HPTs associated with the other three loci – sphB2 (BP1660), tcfA (BP1201), and bapC (BP2738) – are located within the coding sequence. Because HPTs shorter than the threshold lengths used here have been shown to be phase-variable in B. pertussis (e.g., bvgS), three HPT loci with fewer than 9 C/G base pairs were also chosen for analysis (Table 2). HPTs in BP3651 and BP0059 are polymorphic among the sequenced genomes, and the third, upstream of ptxA, is not (Table 2). The HPTs of these nine loci were genotyped by PCR/LDR in a collection of 90 B. pertussis strains encompassing a considerable degree of temporal (70 years) and geographic (4 continents) diversity (Additional file 6).
Table 2.
Additional B. pertussis, B. bronchiseptica, and B. parapertussis HPTs analyzed in this study
| B. pertussis Tohama I | B. bronchiseptica RB50 | B. parapertussis 12822 | ||||||||||
| HPT numa | ORFb | Allelec | ORF | Allele | ORF | Allele | Gene | Base | Poly-morphicd | Loce | Promoter overlapf | Functional categoryf |
| 47 | BP0059 | 8 | BB0530 | 6 | BPP0525 | 6 | C/G | y | C | Global regulatory functions | ||
| 81 | BP1877 | 6 | BB2995 | 6 | BPP3029 | 6 | bvgS | C/G | n | C | Global regulatory functions | |
| 57 | BP3651 | 8 | BB0066 | 6 | BPP0066 | 6 | C/G | y | I | H | Cell envelope | |
| 85 | BP3783 | 6 | BB4890 | 6 | BPP4304 | 6 | ptxA | C/G | n | I | K | Periplasmic/exported/lipoproteins |
a HPT number is an arbitrarily assigned unique ID. Table is sorted by order in the B. bronchiseptica RB50 genome.
b Identifier of ORF that is either contains or is downstream of the HPT. Empty cell indicates that no orthologous sequence exists in that genome.
c Numbers are length of HPTs. Sequences are given when the tract contains a substitution relative to alleles in the other genomes.
d "y" denotes HPT length polymorphism among the three sequenced genomes.
e "I", located in intergenic region; "C", located in coding sequence
f Probability that the HPT overlaps a promoter coded as follows: H, high; K, known to overlap experimentally defined promoter.
Values were determined only for coding HPTs. See Methods for definitions of classes.
g Functional categories from MultiFun classification system as assigned by Parkhill, et al. [3]
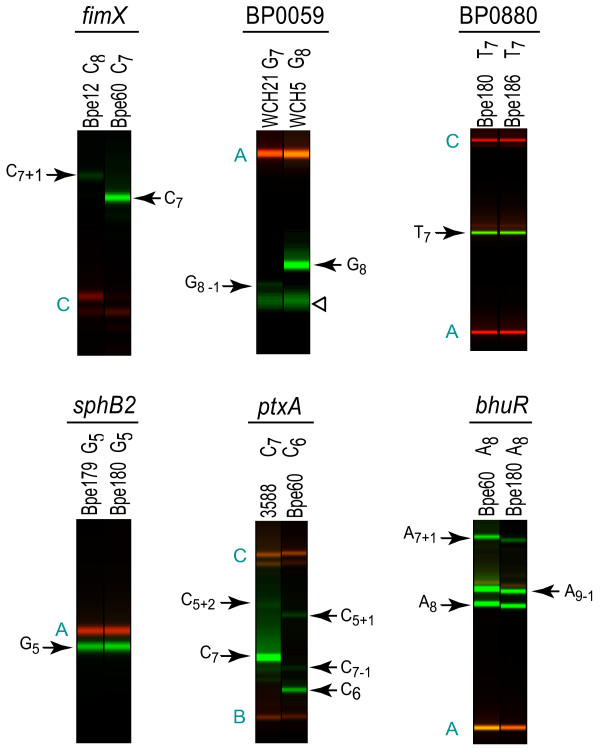
If Tohama I were a representative B. pertussis strain, variant alleles of each HPT in other strains would be most often either one base pair longer or shorter than the Tohama I allele. Thus, for each locus, three discriminating oligonucleotides were synthesized: one each targeting the HPT in the Tohama I allele, the -1 allele (relative to Tohama I), and the +1 allele (Additional file 7). The BP3651, fimX, BP0880, BP0059 and sphB2 HPTs were screened with uniplex reactions that contained the single discriminating oligonucleotide that targets the Tohama I allele, and the FAM-labeled common oligonucleotide. The remaining HPTs were screened by multiplex PCR/LDR reactions in which all three discriminating oligonucleotides were mixed with a FAM-labeled common oligonucleotide. Strains yielding PCR/LDR profiles different from Tohama I were further analyzed by sequencing the PCR product containing the variable HPT. In cases where no ligation products were detected, HPT length was determined by sequencing.
Among the strains examined, the HPTs in BP0880, sphB2, and bhuR were not polymorphic, indicating that they are stable in the B. pertussis population (Figure 3; Additional files 8, 9, and 10). Two HPTs varied in only a single B. pertussis strain. The coding HPT in BP0059 contained one additional base in WCH21, an isolate from Australia (Figure 3 and Additional file 11). This HPT is the site of a frameshift mutation that truncates the ORF in B. pertussis Tohama I. The single base insertion in WCH21 restores the reading frame and is predicted to result in a full-length protein; all other strains assayed carry the frameshifted allele. The HPT upstream of ptxA varied by the addition of a single base in strain 03588, isolated in Italy in 1993 (Figure 3 and Additional file 12). This insertion increases the distance between the predicted -35 and -10 boxes of the ptx-ptl operon [33]. Changing the distance between these regulatory elements by as few as two bases has been shown to reduce dramatically transcription of this key toxin expression locus, suggesting that the HPT variation in 03588 may have significant consequences for expression [34].
Figure 3.
Representative PCR/LDR results for HPTs in fimX, BP0059, BP0880, sphB2, ptxA, and bhuR. Raw capillary electrophoresis data for ligation products (green) and molecular weight standards (red) displayed as if an electrophoretic gel image. Assayed locus is indicated at the top of each panel; strain and length of its HPT allele are noted above each lane. Representative LDR results are depicted here; for complete data from all strains see Additional files 8, 9, 10, 11, 12, and 13. BP0880, BP0059, fimX, and sphB2 HPTs were screened by uniplex LDR reactions containing only the discriminating oligonucleotide that targets the Tohama I sequence. The ptxA and bhuR HPTs were screened by multiplex reactions that contain all three discriminating oligonucleotides. Letters in teal indicate molecular weight standards as labeled in the corresponding supplementary figures. Ligation products (arrows) are labeled using the notation described in the legend to Figure 1. The open arrowhead indicates a synthesis artifact in the BP0059 common oligonucleotide preparation.
The five HPTs described above maintained a constant length over the duration of each B. pertussis batch culture experiment (representing 109 bacteria). Since the detection limit of the assay is 1 variant allele in 100, the proportion of mutants after 30 [log2(109)] generations has an upper bound of 0.01. Using the mathematical model developed by Saunders, et al. [35], the maximum phase variation rate for these loci, assuming equal forward and back mutation rate and no fitness difference, is 6.7 × 10-4 per generation.
HPT variation in fimX and other fimbrial major subunit gene promoters
In seven strains, the HPT upstream of fimX differed from the Tohama I allele (C7) by the addition of a single base (Figure 3; Additional files 13 and 14). After excluding replicate isolates, four independent strains with the longer allele remained. This HPT is also known as the "C-stretch" motif of a previously identified fimbrial gene promoter element, which also contains the conserved "fim box" [26,27]. The length of the C-stretch in the fim2 and fim3 promoters has been shown to influence transcriptional activity [26,27]. Furthermore, deliberate mutation of the C-stretch upstream of fimX affected transcription of that gene [26]. In order to examine whether fimX expression differed between the allelic variants in our collection, quantitative RT-PCR was used to assay fimX transcript abundance in a sample of strains harboring C8 versus C7 tracts. Linear regression analysis showed a strong correlation (R2 = 0.611) between transcript abundance and tract length, in which strains with the C8 HPT had an average fimX transcript abundance 3.8-fold lower than those with a C7 HPT (p = 0.0137) (Additional file 14). Although this result strongly suggested that a single base length polymorphism in this HPT may influence fimX expression, differential effects due to cis- or trans-regulatory factors cannot be ruled out. However, the sequences of the 64 bases upstream of the C-stretch and 87 bases downstream, including the translational start site, did not differ between the strains examined. Among the 51 independent clinical isolates from the United States and the Netherlands for which vaccine history was known, all three of the C8 fimX HPT alleles were from strains isolated prior to the introduction of vaccination (1949 in the United States, and 1953 in the Netherlands; two-tailed p = 0.0146, Fisher's exact test).
Other B. pertussis and B. bronchiseptica fimbrial major subunit genes exhibit allelic polymorphisms in coding regions [36-38] and in the long cytosine HPTs (up to C19 in B. bronchiseptica fim3) in their promoters [26,27,39]. Because the data above suggest a shift in fimX HPT alleles subsequent to vaccine introduction, the HPTs upstream of fim2 and fim3 were assayed for evidence of a concomitant change. Because HPTs greater than 16 nt are not reliably measured by LDR [28], these HPTs were characterized by direct sequencing of PCR products, recognizing that the generation of slippage products in the PCR and sequencing reactions remains a potential source of experimental error.
HPT lengths for fim2 ranged in this set of B. pertussis strains from 10 to 15. As with fimX HPT length polymorphisms, the pre-vaccine strains (median = 13.5) had significantly longer fim2 HPTs than post-vaccine strains (median = 11; p = 0.0339, Mann-Whitney U-test; Additional file 14). Likewise, HPT lengths for fim3 ranged in this sample from 13 to 18; pre-vaccine strains (median = 14.5) tended to have longer tracts than post-vaccine strains (median = 13), but this difference did not achieve statistical significance (p = 0.0771, Mann-Whitney U-test).
HPTs that varied during a single round of culture
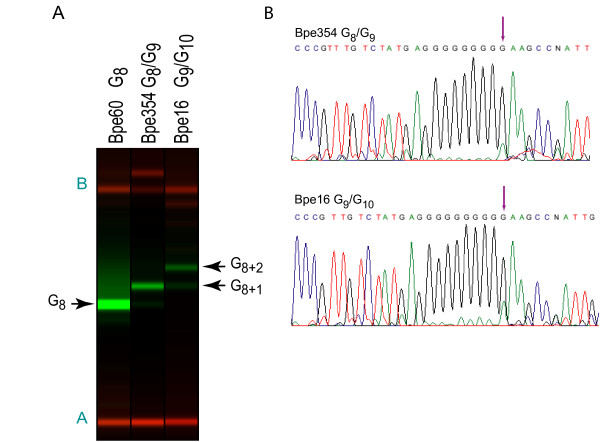
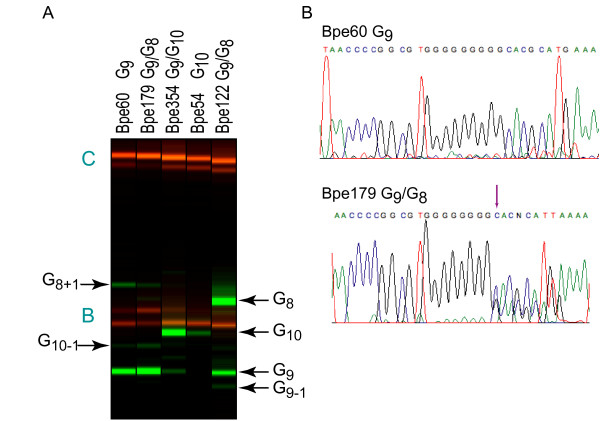
The HPTs of tcfA, bapC, and BP3651 exhibited tract length variation between strains, and within genomic DNA samples derived from cultures of 109 bacteria grown from single colonies over 30 [log2(109)] generations (Additional file 15). Variant alleles of the HPT upstream of BP3651 were found in six strains (Figure 4; Additional files 15 and 16). The atypical B. pertussis strain, 18323, represented by three separate isolates, harbored a mixture of G9 and G10 alleles. Three other independently isolated strains displayed a mixture of G8 and G9 alleles. In all other strains in this collection, only the G8 allele was detected, suggesting that this HPT may be unstable only when its length exceeds a critical threshold of 8 bases. In order to determine whether allelic variation was present in smaller populations founded by a single cell, five independent single colonies (approximately 107 bacteria; data not shown) of two variable strains, Bpe354 and Bpe16, were assayed by colony PCR/LDR. In all cases, each of the single colonies yielded mixed LDR profiles, indicating that allelic variation in the BP3651 HPT arises over the approximately 23 [log2(107)] generations required to produce a macroscopic colony.
Figure 4.
Representative PCR/LDR data for the BP3651 HPT. (A) Raw capillary electrophoresis data for ligation products from BP3651 uniplex PCR/LDR (green) and molecular weight standards (red) displayed as if an electrophoretic gel image. Strains are indicated above each lane. Ligation products (arrows) are labeled using the notation described in the legend to Figure 1. Letters in teal indicate molecular weight standards as labeled in Additional file 16. (B) Partial traces from direct sequencing of BP3651 PCR products. Strain and alleles are noted above each sequence trace. Purple arrows indicate the point in the sequence trace after which peak shadowing, indicative of a mixture of HPT alleles, can be observed.
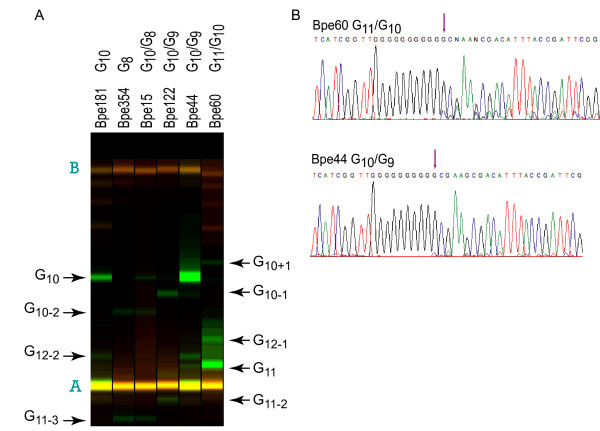
The predominant bapC HPT allele in 76/90 isolates was G11, but almost all of these also harbored a detectable fraction of G10 and G12 alleles (Figure 6 and Additional file 18). Six strains carried primarily the G10 allele, two carried primarily the G9 allele, and four carried primarily the G8 allele, but in most of these cases, a mixture of alleles was detected. In many cases, mixed allelic content was verified by sequencing (Figure 6). In order to assess allelic variation in small populations founded by a single cell, 93 independent Bpe60 colonies, each containing approximately 1 × 107 bacteria, were assayed by colony PCR/LDR. In every case, multiple alleles were detected, with the G11 allele predominating, but G10, G12, and in some cases G13 alleles also present (Additional file 19), indicating that allelic variation in the bapC HPT also arises within 23 generations. The G11 tract, as annotated in the Tohama I genome sequence, causes a shift in the reading frame that is predicted to result in a truncated BapC protein. However, a single base contraction of the HPT would restore the reading frame, leading to synthesis of a full-length protein.
Figure 6.
Representative PCR/LDR data for the bapC HPT. Raw capillary electrophoresis data for ligation products from bapC multiplex PCR/LDR (green) and molecular weight standards (red) displayed as if an electrophoretic gel image. Strains and HPT allele lengths are indicated above each lane. Ligation products (arrows) are labeled using the notation described in the legend to Figure 1. Letters in teal indicate molecular weight standards as labeled in Additional file 18. (B) Representative traces from direct sequencing of bapC PCR products. Strain and alleles are noted above each sequence trace. Purple arrows indicate the point in the sequence trace after which peak shadowing, indicative of a mixture of HPT alleles, can be observed.
The predominant tcfA allele in 82/90 strains was G9, but the G8 allele was detected as a minor allele in 75 of these (Figure 5 and Additional file 17). One strain, Bpe122, yielded approximately equal numbers of G8 and G9 alleles. Five strains carried primarily the G10 allele, but four of these also harbored a detectable fraction of G9 and G11 alleles. One- or two-base insertions into the G9 HPT, beginning at codon 130 of the tcfA ORF, alter the reading frame, and presumably result in a truncated, non-functional Tcf protein. Stability of the tcfA HPT was further assessed by cloning PCR products containing the HPT into a plasmid in E. coli. Eight independent E. coli clones harboring PCR products from the Bpe354 genomic DNA preparation, which carried predominantly G10 alleles, but detectable G9 alleles, were assayed by colony PCR/LDR. Five of the clones carried a mixture of alleles similar to the original Bpe354 genomic DNA preparation, while three clones carried a mixture of G10 and G11 alleles, indicating that allelic contraction and expansion at this locus occurs in E. coli (data not shown).
Figure 5.
Representative PCR/LDR data for the tcfA HPT. (A) Raw capillary electrophoresis data for ligation products from tcfA multiplex PCR/LDR (green) and molecular weight standards (red) displayed as if an electrophoretic gel image. Strains are indicated above the lane. Ligation products (arrows) are labeled using the notation described in the legend to Figure 1. Letters in teal indicate molecular weight standards as labeled in Additional file 17. (B) Representative traces from direct sequencing of tcfA PCR products. Strain and alleles are noted above each sequence trace. Purple arrow indicates the point in the sequence trace after which peak shadowing, indicative of a mixture of HPT alleles, can be observed.
Discussion
B. pertussis is unusual among bacterial pathogens in its limited genomic diversity. Multiple methods, including MLST, MLEE, and CGH, have found that B. pertussis is highly clonal with very few genomic differences, even among temporally and geographically diverse strains [4,10,11]. Phase variation could be an important mechanism in B. pertussis for generation of phenotypic diversity in the absence of genome plasticity. In fact, prior to this study, a small number of key virulence phenotypes of B. pertussis were already known to be phase-variable.
This study focused on the identification and characterization of HPTs, because they are prone to expansion and contraction and often associated with bacterial phase-variable loci. Evidence of phase variation can be obtained by demonstrating repeat length polymorphism within multiple genomes from the same species, or closely related species as in this study. Scanning multiple related genomes has the added benefit of identifying polymorphic, putative, phase-variable loci that fail to meet the identification threshold in one strain but meet it in another. For example, if only the TohamaI genome had been scanned, 40 HPTs would have been identified,; however, the comparative approach identified 12 more HPTs that are shorter than the threshold in B. pertussis but meet the threshold in at least one of the closely related Bordetella genomes.
The comparative approach, while identifying HPTs that have changed at least once since the divergence of the two genomes, does not address the key issue of allelic variation rate. Are these differences stable over the long time periods that differentiate bacterial lineages, or do they fluctuate rapidly, with each genome sequence providing only a snapshot of the genome at a specific point in time? An estimate of HPT variation frequency can be obtained by measuring repeat length in multiple genomes, and doing so with a method sensitive enough to detect rare variants in a mixture of alleles. PCR/LDR is an ideal method for detecting rare genotypes as it can clearly delineate numerous genotypes within a mixed sample. While the assay is not quantitative, its sensitivity allows detection of one variant HPT in 100 ([28]; this study). With a sequencing-based approach, assuming a Poisson distribution, 300 clones would need to be analyzed to have a 99% chance of detecting a variant allele representing 1% of the sample.
The frequency of allelic variation differed greatly among the nine HPTs genotyped in this study. Three HPTs were invariant among the 90 B. pertussis isolates, two differed in a single strain, and one differed between strains isolated during different eras. Because no single genomic DNA preparation harbored a detectable mixture of HPT alleles, a maximum mutation rate of 6.7 × 10-4 mutations per generation was inferred for these six loci. The actual mutation rate could be significantly lower, but accurate determination would require identification of cultures containing variant alleles, and estimation of the relative fitness of each genotype, both of which are impractical due to the low apparent rate of variation and lack of a known selectable phenotype. Three HPTs exhibited allelic polymorphism within single colonies. The HPT in BP3651 was variable within a single culture only in strains with alleles longer than C8, leading us to speculate that an HPT might become relatively unstable with the addition of a single base. The mechanisms that regulate the frequency of HPT phase variation are not clearly understood, but conditions that influence the activity of the mismatch repair pathway have been shown to affect the frequency of phase variation [40]. Although the mutation rate at the BP3651, tcfA, and bapC HPTs is apparently much higher than for the other HPTs examined here, accurate determination of these rates would require quantitative estimates of the proportion of each allele, which is not possible using LDR. The relative fitness of each genotype is also a key parameter in this calculation [35], but because stable genotypes cannot be isolated in culture, these measurements cannot easily be made. However, fitness differences would reduce frequency of deleterious alleles, making the mutation rate appear lower than it actually is.
The discovery here of a B. pertussis strain with an expanded HPT in a region of the ptx promoter known from mutational studies to be critical for transcriptional activity [34] suggests that ptxA expression may be atypical in this strain. Although numerous studies have documented allelic variation in the coding region of ptxA (e.g., [36,41,42]), only one study focused on potential regulatory polymorphisms in the ptx promoter region, identifying rare single nucleotide polymorphisms further upstream of ptxA, but not in the HPT [43]. Allelic shifts subsequent to the introduction of vaccination in the ptxA coding region support a model of vaccine-driven evolution [44]. Perhaps modified expression of PT could also confer a fitness advantage in a vaccinated host population. These possibilities deserve and require further investigation.
Two genes encoding autotransporter family proteins had the most highly variable HPTs among the loci assayed. The tcfA gene encodes tracheal colonization factor (Tcf), a secreted protein produced by B. pertussis [45]. Disruption of this gene resulted in a 10-fold reduction in colonization of a mouse model, suggesting that it is an important virulence determinant [45]. The phase-variable HPT is located in the tcfA open reading frame such that expansions and contractions of the tract would result in the reversible truncation, and presumed inactivation, of the Tcf protein product. This HPT is the location of the frameshift polymorphism previously identified in the tcfA5 allele, which was proposed to represent a tcfA phase variant [41]. The PCR/LDR results suggest that, within a culture of B. pertussis, only a fraction of cells express full-length TcfA. The prevalence of antibodies against TcfA in human populations has not been addressed, but the existence in the B. pertussis population of multiple tcfA alleles that encode different protein variants suggests that it may be under selection by immune surveillance [41]. If so, B. pertussis cells that express Tcf in vivo might be more rapidly cleared by the immune system, and cells that do not express Tcf or express a variant form, as a result of phase variation, would have a selective advantage. However, through the continuous emergence of Tcf-expressing phase variants within the population, production of this secreted virulence factor could be maintained in temporal phases or niches where the benefits of expressing it outweigh the risk of increased susceptibility to immune clearance.
Frequent phase variation was also observed at the HPT in the coding region of the bapC gene. By analogy to other B. pertussis autotransporter family proteins that have been implicated in pathogenesis, a role for this gene in virulence has been proposed [46], but such a function has not yet been demonstrated. Likewise, nothing is known about the immune response to BapC, but its predicted localization on the cell surface makes it a plausible target for immune surveillance. In a previous study, partial sequencing of bapC from 10 B. pertussis strains identified only a silent SNP [46], but the 5' end of the gene, which contains the HPT, was not examined. Interestingly, bapC harbors a second HPT (C14 in Tohama I), upstream of the HPT assayed here, that may also be phase-variable (Table 1).
By characterizing fimX allelic polymorphism and gene expression, this study expands upon previous work on phase variation of fimbrial major subunit genes. Among the strains examined, seven were found to harbor a C8 HPT in the fimX promoter region, distinguishing them from the majority of strains which carried a C7 tract. fimX transcript abundance was found to be nearly fourfold higher in C7 strains than in C8 strains, suggesting that this single base difference may significantly influence transcription, and arguing against the model in which fimbrial gene expression is only activated when the distance between the activator binding region and the -10 box exceeds a threshold of 22 bases [26,27]. The data presented here suggest that the activity of these promoters may be more subtly regulated by HPT expansions and contractions, even when the tract is relatively short.
The C8 allele of the HPT in the fimX promoter was found only in B. pertussis strains isolated prior to the introduction of pertussis vaccines, suggesting that vaccination may have led to selection against strains carrying this allele. Because the C8 HPT correlates with decreased fimX transcript levels, we propose that increased FimX expression may confer a selective advantage in an immunized host population. In B. pertussis populations from multiple countries, initiation of a vaccination program using a Fim2-expressing vaccine strain has led to the significant enrichment of Fim3-expressing isolates (reviewed in [38]. By analogy, vaccine-induced selection against Fim2 may have also led to the enrichment of strains that express more FimX. Such a shift would not have been detected by serotyping because only Fim2 and Fim3 antisera have been used in these assays. The substitution of one fimbrial subtype for another has been proposed as a mechanism to evade the immune system without substantial loss of adhesion [47].
Conclusion
Computational screening was used to identify significantly long HPTs that were predicted to be prone to length polymorphism in the genomes of three closely related Bordetella species. A comparative genomic approach verified that many of these loci are, in fact, polymorphic among the three species. In order to obtain evidence for HPT length polymorphism within a single species, nine HPT loci were genotyped using the PCR/LDR assay in a collection of 90 B. pertussis strains. Polymorphism was observed at six of these loci, suggesting that a thorough examination of all 52 B. pertussis HPTs could reveal dozens of previously unrecognized phase-variable genes. Three HPTs displayed tract length polymorphism within cultures derived from single colonies, indicating that they are rapidly phase-variable. A fourth polymorphic HPT was identified in the promoter of fimX where a single base expansion variant was present only among strains that were isolated prior to introduction of pertussis vaccines. Transcript abundance of fimX was found to be 3.8-fold lower in strains carrying the longer allele.
Given the diversity of alternate phenotypes that may be generated by the 69 HPTs identified in this study, it is likely that phase variation is involved in many aspects of Bordetella pathogenesis. Because B. pertussis lacks genetic diversity, phase variation may be a key mechanism for adaptation to the hostile and changing host environment. Future work to elucidate functional differences associated with the phase variants described in this study may lead to new insights into Bordetella host specificity, immune system modulation, and niche adaptation.
Methods
Strains, media, and genomic DNA preparation
Strains used in this study are described in Additional file 6. Pre-vaccination era strains in the U.S. were defined as strains isolated before 1949, when use of the whole-cell pertussis vaccine became widespread [48]. U.S. strains isolated subsequent to 1949 were considered to be post-vaccination era strains. Strains from the Netherlands were considered pre-vaccination era strains if isolated prior to 1953, and post-vaccination era strains if isolated after 1953 [37]. Strains from Australia and Italy were not included in the statistical analysis of fimX polymorphisms, as pre- and post-vaccination status for each strain was not available.
Single B. pertussis colonies from Bordet-Gengou agar (BD, Franklin Lakes, New Jersey, United States) plates supplemented with 15% defibrinated sheep blood (BG blood plates) were inoculated into modified Stainer-Scholte media (SS) and grown to stationary phase. Genomic DNA was purified from bacterial cell pellets using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) as previously described [2].
Homopolymeric tract identification and statistical analysis
HPTs were identified by scanning Bordetella genome sequences (RefSeq NC_002927, NC_002928, and NC_002929) using the equicktandem program of the EMBOSS package [49]. Markov model transition probabilities and observed number of HPTs were calculated using calc_transition_matrices.pl and count_HPTs.pl (available at [50]). For C or G HPTs, second through fifth order Markov models yielded very similar observed/expected curves, and for A or T HPTs, first and second order Markov models yielded very similar observed/expected curves, but higher order Markov models may exhibit overfitting. Orthologous HPTs in the three Bordetella genomes were manually confirmed using ACT [51], and alignments were verified using cross_match [52] and ClustalW [53]. Routine statistical analyses were performed using StatView v. 5.0.1 software (SAS Institute, Cary, NC, USA).
The position of each intergenic HPTs was mapped relative to adjacent genes in order to determine whether it could influence transcriptional regulation. Because most Bordetella promoters have not been functionally characterized, we made the assumption that the distribution of distances between promoter and start codon would follow a similar trend to the distribution found in the E. coli genome [54]. Extrapolating from this distribution, HPTs upstream of at least one gene were considered highly likely to overlap a promoter element when located between 21 and 80 nt upstream of the start codon of an ORF. HPTs located fewer than 21 nt, or between 81 and 150 nt upstream of the start codon were considered to have a moderate likelihood of overlapping a promoter, while those more than 150 nt upstream were considered to have a low probability. HPTs between adjacent, convergently transcribed ORFs were assumed not to overlap a promoter.
PCR, cloning and sequencing
All PCR amplifications, unless otherwise indicated, employed Pfu Ultra or Pfu Turbo High Fidelity DNA polymerase (Stratagene, La Jolla, CA, USA) according to the manufacturer's instructions. Fidelity comparison experiments used AmpliTaq DNA Polymerase (Ambion, Austin, TX, USA). 25 μl reaction volumes contained 25 ng of genomic DNA, 10 mM dNTPs, and 20 pmol of each oligonucleotide primer (see Additional file 20 for PCR primers used). PCR parameters were 5 min at 96°C; followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1 min; and a final 7 min extension step at 72°C. Reactions were then heated for 30 minutes at 96°C to inactivate the polymerase. 5 μl of the PCR reaction was visualized on a 2% agarose gel to confirm the presence of an amplification product of the expected size. Selected PCR products were sequenced directly (Elim Biopharmaceuticals, Hayward, CA, USA) using one amplification primer. PCR oligonucleotide primer (Integrated DNA Technologies, Coralville, IA, USA) sequences are in Additional file 20.
Some PCR products were cloned as blunt-ended molecules using the Zero Blunt TOPO PCR Cloning Kit and the pCR II-Blunt TOPO vector (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions, with the following modifications to maximize efficiency when cloning a pool of PCR products: 4 μl of PCR product, 1 μl Salt Solution and 1 μl vector were incubated for 20 minutes; 4 μl of the cloning reaction was added to a vial of One Shot Chemically Competent E. coli, which was then gently mixed and incubated on ice for 5 minutes.
Colony PCR of B. pertussis strains was performed on pinprick size colonies grown on BG blood plates at 37°C by resuspending a single colony in 10 μl of 1× Pfu Ultra High Fidelity Polymerase Buffer (New England BioLabs, Ipswitch, MA, USA), and incubating at 96°C for 10 minutes. The resulting cell lysate was centrifuged at 600 × g for 1 min. 2 μl of the cleared lysate was used as the genomic template for subsequent PCR reactions as described above.
LDR conditions
Uniplex PCR/LDR reactions contained 1 μl of a PCR reaction, 25 nmol of labeled common oligonucleotide, and 25 nmol of one unlabeled discriminating oligonucleotide. Multiplex PCR/LDR reactions contained 1 μl of PCR reaction mix, 25 nmol of labeled common oligonucleotide, and 25 nmol each of three different unlabeled discriminating oligonucleotides. All LDR oligonucleotides were synthesized and HPLC purified by Integrated DNA Technologies (see Additional file 7). All PCR/LDR reactions were performed in 1× Tth DNA Ligase Buffer (New England BioLabs) at a final volume of 20 μl. The reaction mixture was heated for 1.5 min at 94°C prior to adding 25 fmol of Tth DNA Ligase (New England BioLabs). LDR reactions were cycled 30 times, each cycle consisting of 15 s at 94°C and 2 min at 65°C. Reactions were stopped with 1.5 μl of 0.5 mM EDTA, then spiked with a custom mixture of fluorescent 2',7'-dimethoxy-4',5'-dichloro-6-carboxyfluorescein (JOE)-labeled oligonucleotide molecular weight standards (Integrated DNA Technologies) at 100 fmol. Reactions were analyzed using an ABI 3100 capillary sequencing instrument (Applied Biosystems, Foster City, CA, USA) at the Stanford Protein and Nucleic Acid Biotechnology Facility (Stanford, CA, USA). In cases where no ligation product was detectable, PCR products were sequenced directly to determine HPT length.
LDR data analysis
Raw trace data from ABI format output files were displayed as images using gel_draw.pl. Molecular weights of ligation products were calculated from raw trace data by comparing their mobility to that of oligonucleotide standards using ABImulti.py. Both of these scripts utilize the convert_trace and scf_dump programs of the Staden package [55] for extraction of raw fluorescence intensity data from ABI format output files. Software described in this manuscript is available for download at [50].
Measurements of gene expression
Cultures of B. pertussis were established by inoculating a single colony from a BG blood plate into 1.5 ml of SS, incubating at 37°C with shaking until an OD600 of approximately 2 was achieved, and sub-cultured, in duplicate, to an OD600 of 0.05 in SS. These duplicate cultures were then incubated at 37°C with shaking until aliquots were harvested for RNA extraction during mid-log phase (OD600 1.5 – 2.5). RNA was extracted and reverse transcribed as previously described [56]. Quantitative RT-PCR reactions were carried out in a final volume of 20 μl using IQ Sybrgreen Supermix 2× Mix for Real-time PCR (Bio-Rad Laboratories, Hercules, CA, USA), 1 μl cDNA (in RT reaction mix), 1 μl DMSO, and 6 pmol each forward and reverse primers (Integrated DNA Technologies; see Additional file 20). Real-time PCR was performed with an ABI Prism 7900 HT sequence detection system (Applied Biosystems). Data were analyzed using the ΔΔCt method with recA as the reference [57].
Abbreviations
PCR: polymerase chain reaction
LDR: ligase detection reaction
HPT: homopolymeric tract
PT: pertussis toxin
JOE: 2',7'-dimethoxy-4',5'-dichloro-6-carboxyfluorescein
FAM: 5-carboxyfluorescein
Authors' contributions
EBG designed, performed, and analyzed molecular assays, and drafted the manuscript. CAC conceived of the study, participated in its design, performed bioinformatics and statistical data analysis, developed LDR visualization software, and drafted the manuscript. RCB developed software tools for LDR data analysis. DAR helped in the design of the study, guided its execution, and contributed to interpretation of the data, and preparation of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Supplementary Figure 1. Capillary electrophoresis traces of HPLC-purified bvgS LDR oligonucleotides.
Supplementary Figure 2. Fidelity of Pfu and Taq DNA polymerases over multiple rounds of PCR/LDR.
Supplementary Table 1. Characteristics of B. bronchiseptica HPTs
Supplementary Table 2. Characteristics of B. pertussis HPTs
Supplementary Table 3. Characteristics of B. parapertussis HPTs
Supplementary Table 4. Characteristics of B. pertussis strains used in this study
Supplementary Table 5. LDR and molecular weight standard oligonucleotides
Supplementary Figure 3. BP0880 PCR/LDR.
Supplementary Figure 4. sphB2 PCR/LDR.
Supplementary Figure 5. bhuR PCR/LDR.
Supplementary Figure 6. BP0059 PCR/LDR.
Supplementary Figure 7. ptxA PCR/LDR.
Supplementary Figure 8. fimX PCR/LDR.
Supplementary Table 6. Lengths of HPTs in fim gene promoters and relative expression of fimX transcript.
Supplementary Table 7. Lengths of HPT alleles detected by PCR/LDR for three hypervariable HPT loci.
Supplementary Figure 9. BP3651 PCR/LDR.
Supplementary Figure 10. tcfA PCR/LDR.
Supplementary Figure 11. bapC PCR/LDR.
Supplementary Figure 12. Bpe60 colonies screened for bapC HPT length
Supplementary Table 8. PCR and RT-PCR oligonucleotide primers.
Acknowledgments
Acknowledgements
We are very grateful to Elies Bik and Mary Brinig for their technical and intellectual advice and assistance. We thank Francis Barany (Cornell University, Ithaca, NY, USA) for sharing his expertise in PCR/LDR, Peggy Cotter (University of California-Santa Barbara, CA, USA) for stimulating discussions, and Tim Andriessen and Shripa Patel in the Stanford Protein and Nucleic Acid Biotechnology Facility for expert technical assistance with capillary electrophoresis. For their contributions of B. pertussis strains, we thank Frits Mooi (Rijksinstituut vor Volksgezondheid en Milieu, Bilthoven, The Netherlands), Scott Stibitz, (U.S. Food and Drug Administration, Rockville, MD, USA), Sylvia Yeh (Harbor-UCLA Medical Center, Torrance, CA, USA), Gary Sanden (Centers for Disease Control and Prevention, Atlanta, GA, USA), Maria Li (San Mateo County Public Health Lab, San Mateo, CA, USA), Jeff F. Miller (University of California-Los Angeles, CA, USA), Andrew Lawrence (Women's and Children's Hospital, North Adelaide, Australia), Paola Stefanelli (Istituto Superiore di Sanità, Rome, Italy); and Larry Stauffer and Tasha Poissant (Oregon State Public Health Laboratory, Portland, OR, USA). This work was supported by NIH grants AI057188 and AI054970 to DAR.
Contributor Information
Emily B Gogol, Email: emily.gogol@ucsf.edu.
Craig A Cummings, Email: craigc@stanford.edu.
Ryan C Burns, Email: rburns@stic.net.
David A Relman, Email: relman@stanford.edu.
References
- World Health Organization Pertussis vaccines--WHO position paper. Wkly Epidemiol Rec. 2005;80:31–39. [PubMed] [Google Scholar]
- Cummings CA, Brinig MM, Lepp PW, van de Pas S, Relman DA. Bordetella Species Are Distinguished by Patterns of Substantial Gene Loss and Host Adaptation. J Bacteriol. 2004;186:1484–1492. doi: 10.1128/JB.186.5.1484-1492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL, Cerdeno-Tarraga AM, Temple L, James K, Harris B, Quail MA, Achtman M, Atkin R, Baker S, Basham D, Bason N, Cherevach I, Chillingworth T, Collins M, Cronin A, Davis P, Doggett J, Feltwell T, Goble A, Hamlin N, Hauser H, Holroyd S, Jagels K, Leather S, Moule S, Norberczak H, O'Neil S, Ormond D, Price C, Rabbinowitsch E, Rutter S, Sanders M, Saunders D, Seeger K, Sharp S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Unwin L, Whitehead S, Barrell BG, Maskell DJ. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi FR, van Loo IH, King AJ. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis. 2001;7:526–528. doi: 10.3201/eid0707.017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin SA. Pertussis--a disease and vaccine for all ages. N Engl J Med. 2005;353:1615–1617. doi: 10.1056/NEJMe058181. [DOI] [PubMed] [Google Scholar]
- Elomaa A, Advani A, Donnelly D, Antila M, Mertsola J, Hallander H, He Q. Strain variation among Bordetella pertussis isolates in finland, where the whole-cell pertussis vaccine has been used for 50 years. J Clin Microbiol. 2005;43:3681–3687. doi: 10.1128/JCM.43.8.3681-3687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallander HO, Advani A, Donnelly D, Gustafsson L, Carlsson RM. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J Clin Microbiol. 2005;43:2856–2865. doi: 10.1128/JCM.43.6.2856-2865.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Boursaux-Eude C, Coralie G, Caro V, Guiso N. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. J Clin Microbiol. 2001;39:4396–4403. doi: 10.1128/JCM.39.12.4396-4403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee A, Mooi F, Van Embden J, Musser J. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinig MM, Cummings CA, Sanden GN, Stefanelli P, Lawrence A, Relman DA. Significant gene order and expression differences in Bordetella pertussis despite limited gene content variation. J Bacteriol. 2006;188:2375–2382. doi: 10.1128/JB.188.7.2375-2382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrindt U, Hacker J. Whole genome plasticity in pathogenic bacteria. Curr Opin Microbiol. 2001;4:550–557. doi: 10.1016/s1369-5274(00)00250-2. [DOI] [PubMed] [Google Scholar]
- Hallet B. Playing Dr Jekyll and Mr Hyde: combined mechanisms of phase variation in bacteria. Curr Opin Microbiol. 2001;4:570–581. doi: 10.1016/s1369-5274(00)00253-8. [DOI] [PubMed] [Google Scholar]
- van der Woude MW, Baumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun L, Snyder LA, Saunders NJ. Adaptation by phase variation in pathogenic bacteria. Adv Appl Microbiol. 2003;52:263–301. doi: 10.1016/s0065-2164(03)01011-6. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Meyer TF. 'Small' talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr Opin Microbiol. 2003;6:43–49. doi: 10.1016/s1369-5274(03)00004-3. [DOI] [PubMed] [Google Scholar]
- van Belkum A, Scherer S, van Alphen L, Verbrugh H. Short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev. 1998;62:275–293. doi: 10.1128/mmbr.62.2.275-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;4:203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Salaun L, Ayraud S, Saunders NJ. Phase variation mediated niche adaptation during prolonged experimental murine infection with Helicobacter pylori. Microbiology. 2005;151:917–923. doi: 10.1099/mic.0.27379-0. [DOI] [PubMed] [Google Scholar]
- Jordan PW, Snyder LA, Saunders NJ. Strain-specific differences in Neisseria gonorrhoeae associated with the phase variable gene repertoire. BMC Microbiol. 2005;5:21. doi: 10.1186/1471-2180-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- Weiss AA, Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984;43:263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboli B, Pedroni P, Cuzzoni A, Grandi G, de Ferra F. Expression of Bordetella pertussis fimbrial (fim) genes in Bordetella bronchiseptica: fimX is expressed at a low level and vir-regulated. Microb Pathog. 1991;10:393–403. doi: 10.1016/0882-4010(91)90084-n. [DOI] [PubMed] [Google Scholar]
- Willems R, Paul A, van der Heide HG, ter Avest AR, Mooi FR. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. Embo J. 1990;9:2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirvi M, Nakayama T, Newman G, McCaffrey T, Paty P, Barany F. Ligase-based detection of mononucleotide repeat sequences. Nucleic Acids Res. 1999;27:e40. doi: 10.1093/nar/27.24.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailovich V, Lapa S, Gryadunov D, Sobolev A, Strizhkov B, Chernyh N, Skotnikova O, Irtuganova O, Moroz A, Litvinov V, Vladimirskii M, Perelman M, Chernousova L, Erokhin V, Zasedatelev A, Mirzabekov A. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips. J Clin Microbiol. 2001;39:2531–2540. doi: 10.1128/JCM.39.7.2531-2540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde D, Lai Y, Sun F, Arnheim N. Taq DNA polymerase slippage mutation rates measured by PCR and quasi-likelihood analysis: (CA/GT)n and (A/T)n microsatellites. Nucleic Acids Res. 2003;31:974–980. doi: 10.1093/nar/gkg178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G, Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985;109:633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool CK, Armstrong SK. Integration of environmental signals controls expression of Bordetella heme utilization genes. J Bacteriol. 2004;186:938–948. doi: 10.1128/JB.186.4.938-948.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques RR, Carbonetti NH. Genetic analysis of pertussis toxin promoter activation in Bordetella pertussis. Mol Microbiol. 1997;24:1215–1224. doi: 10.1046/j.1365-2958.1997.4371792.x. [DOI] [PubMed] [Google Scholar]
- Saunders NJ, Moxon ER, Gravenor MB. Mutation rates: estimating phase variation rates when fitness differences are present and their impact on population structure. Microbiology. 2003;149:485–495. doi: 10.1099/mic.0.25807-0. [DOI] [PubMed] [Google Scholar]
- van Amersfoorth SC, Schouls LM, van der Heide HG, Advani A, Hallander HO, Bondeson K, von Konig CH, Riffelmann M, Vahrenholz C, Guiso N, Caro V, Njamkepo E, He Q, Mertsola J, Mooi FR. Analysis of Bordetella pertussis populations in European countries with different vaccination policies. J Clin Microbiol. 2005;43:2837–2843. doi: 10.1128/JCM.43.6.2837-2843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo IH, Mooi FR. Changes in the Dutch Bordetella pertussis population in the first 20 years after the introduction of whole-cell vaccines. Microbiology. 2002;148:2011–2018. doi: 10.1099/00221287-148-7-2011. [DOI] [PubMed] [Google Scholar]
- Godfroid F, Denoel P, Poolman J. Are vaccination programs and isolate polymorphism linked to pertussis re-emergence? Expert Rev Vaccines. 2005;4:757–778. doi: 10.1586/14760584.4.5.757. [DOI] [PubMed] [Google Scholar]
- Savelkoul PH, de Kerf DP, Willems RJ, Mooi FR, van der Zeijst BA, Gaastra W. Characterization of the fim2 and fim3 fimbrial subunit genes of Bordetella bronchiseptica: roles of Fim2 and Fim3 fimbriae and flagella in adhesion. Infect Immun. 1996;64:5098–5105. doi: 10.1128/iai.64.12.5098-5105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Sun L, Hood DW, Moxon ER. Involvement of genes of genome maintenance in the regulation of phase variation frequencies in Neisseria meningitidis. Microbiology. 2004;150:3001–3012. doi: 10.1099/mic.0.27182-0. [DOI] [PubMed] [Google Scholar]
- van Loo IH, Heuvelman KJ, King AJ, Mooi FR. Multilocus sequence typing of Bordetella pertussis based on surface protein genes. J Clin Microbiol. 2002;40:1994–2001. doi: 10.1128/JCM.40.6.1994-2001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiday P, Sanden G, Heuvelman K, Mooi F, Bisgard KM, Popovic T. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1935-1999. J Infect Dis. 2000;182:1402–1408. doi: 10.1086/315881. [DOI] [PubMed] [Google Scholar]
- Nygren M, Reizenstein E, Ronaghi M, Lundeberg J. Polymorphism in the pertussis toxin promoter region affecting the DNA-based diagnosis of Bordetella infection. J Clin Microbiol. 2000;38:55–60. doi: 10.1128/jcm.38.1.55-60.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi FR, van Oirschot H, Heuvelman K, van der Heide HG, Gaastra W, Willems RJ. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998;66:670–675. doi: 10.1128/iai.66.2.670-675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn TM, Stevens LA. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microbiol. 2004;53:355–365. doi: 10.1099/jmm.0.05515-0. [DOI] [PubMed] [Google Scholar]
- Geuijen CA, Willems RJ, Hoogerhout P, Puijk WC, Meloen RH, Mooi FR. Identification and characterization of heparin binding regions of the Fim2 subunit of Bordetella pertussis. Infect Immun. 1998;66:2256–2263. doi: 10.1128/iai.66.5.2256-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Pertussis--United States, January 1992-June 1995. MMWR Morb Mortal Wkly Rep. 1995;44:525–529. [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Web supplement for "Phase variation and microevolution at homopolymeric tracts in Bordetella pertussis" http://asiago.stanford.edu/PV/ [DOI] [PMC free article] [PubMed]
- Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. ACT: the Artemis Comparison Tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- Green P. cross_match. 1996. http://www.phrap.org/phredphrapconsed.html
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta AM, Collado-Vides J. Sigma70 promoters in Escherichia coli: specific transcription in dense regions of overlapping promoter-like signals. J Mol Biol. 2003;333:261–278. doi: 10.1016/j.jmb.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Staden Package WWW site http://staden.sourceforge.net/
- Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol. 2006;188:1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Capillary electrophoresis traces of HPLC-purified bvgS LDR oligonucleotides.
Supplementary Figure 2. Fidelity of Pfu and Taq DNA polymerases over multiple rounds of PCR/LDR.
Supplementary Table 1. Characteristics of B. bronchiseptica HPTs
Supplementary Table 2. Characteristics of B. pertussis HPTs
Supplementary Table 3. Characteristics of B. parapertussis HPTs
Supplementary Table 4. Characteristics of B. pertussis strains used in this study
Supplementary Table 5. LDR and molecular weight standard oligonucleotides
Supplementary Figure 3. BP0880 PCR/LDR.
Supplementary Figure 4. sphB2 PCR/LDR.
Supplementary Figure 5. bhuR PCR/LDR.
Supplementary Figure 6. BP0059 PCR/LDR.
Supplementary Figure 7. ptxA PCR/LDR.
Supplementary Figure 8. fimX PCR/LDR.
Supplementary Table 6. Lengths of HPTs in fim gene promoters and relative expression of fimX transcript.
Supplementary Table 7. Lengths of HPT alleles detected by PCR/LDR for three hypervariable HPT loci.
Supplementary Figure 9. BP3651 PCR/LDR.
Supplementary Figure 10. tcfA PCR/LDR.
Supplementary Figure 11. bapC PCR/LDR.
Supplementary Figure 12. Bpe60 colonies screened for bapC HPT length
Supplementary Table 8. PCR and RT-PCR oligonucleotide primers.