Abstract
Shortly after the release of singlet oxygen (1O2), drastic changes in nuclear gene expression occur in the conditional flu mutant of Arabidopsis that reveal a rapid transfer of signals from the plastid to the nucleus. In contrast to retrograde control of nuclear gene expression by plastid signals described earlier, the primary effect of 1O2 generation in the flu mutant is not the control of chloroplast biogenesis but the activation of a broad range of signaling pathways known to be involved in biotic and abiotic stress responses. This activity of a plastid-derived signal suggests a new function of the chloroplast, namely that of a sensor of environmental changes that activates a broad range of stress responses. Inactivation of the plastid protein EXECUTER1 attenuates the extent of 1O2-induced up-regulation of nuclear gene expression, but it does not fully eliminate these changes. A second related nuclear-encoded protein, dubbed EXECUTER2, has been identified that is also implicated with the signaling of 1O2-dependent nuclear gene expression changes. Like EXECUTER1, EXECUTER2 is confined to the plastid. Inactivation of both EXECUTER proteins in the ex1/ex2/flu triple mutant is sufficient to suppress the up-regulation of almost all 1O2-responsive genes. Retrograde control of 1O2-responsive genes requires the concerted action of both EXECUTER proteins within the plastid compartment.
Keywords: oxidative stress, retrograde signaling, singlet oxygen, chloroplast
In plants, continuous generation of reactive oxygen species (ROS) is an unavoidable consequence of aerobic metabolic processes such as photosynthesis and respiration that has necessitated the evolution of various scavengers to minimize the cytotoxic impact of ROS on cells. Sensing changes of ROS concentrations that result from metabolic disturbances is being used by plants to evoke stress responses that support plants to cope with environmental variation (1–3). Plants may also produce ROS in a genetically controlled way (e.g., by NADPH oxidases) and use these molecules as signals to control a broad range of processes that comprise defense reactions against pathogens (4), the closure of stomata (5), the regulation of cell expansion and plant development (6), and the control of plant–fungus interactions (7). Chloroplasts and peroxisomes have been shown to be major sites of ROS production (3, 8). The enhanced generation of ROS in these cellular compartments has been attributed to the disturbance of photosynthetic electron transport by a variety of environmental factors (such as high light, high or low temperatures, salt, and drought) that trigger various stress responses (3, 8). One of the difficulties in elucidating the biological activities of ROS during these processes stems from the fact that, in plants under stress, several chemically distinct ROS are generated simultaneously within different intracellular compartments, thus making it very difficult to link a particular stress response to a specific ROS (3, 9). This problem has been alleviated by using the conditional flu mutant of Arabidopsis to study the biological activity of only one of these ROS at a given time (9).
In the dark, the flu mutant accumulates protochlorophyllide (Pchlide), a potent photosensitizer that upon illumination generates singlet oxygen (1O2) (9–11). Immediately after a dark-to-light shift, mature flu plants stop growing, whereas flu seedlings bleach and die. By varying the length of the dark period, one can modulate noninvasively the level of the photosensitizer Pchlide and define conditions that minimize the cytotoxicity of 1O2 and reveal the genetic basis of 1O2-mediated signaling as indicated by the inactivation of the EXECUTER1 gene that is sufficient to abrogate 1O2–dependent stress responses (12). The enhanced generation of 1O2 within plastids that triggers drastic phenotypic changes would be expected to modulate nuclear gene expression as well. Indeed, 2 h after the release of 1O2, rapid changes in the expression of nuclear genes have been shown to affect ≈5% of the total genome of Arabidopsis (9). However, as reported in the present work, inactivation of the EXECUTER1 gene of the flu mutant is not sufficient to fully suppress 1O2-induced changes in nuclear gene expression, suggesting that a residual 1O2-induced transduction of signals from the plastid to the nucleus still operates in the absence of EXECUTER1. We have identified a second signaling component closely related to EXECUTER1 that is also present inside the plastid compartment and, together with EXECUTER1, is required for 1O2-dependent signaling of nuclear gene activities. This protein has been dubbed EXECUTER2. The EXECUTER1 and EXECUTER2 genes are highly conserved among higher plants and thus seem to play an important but hitherto unknown role during the transfer of stress-related signals from the plastid to the nucleus.
Results
Identification and Localization of EXECUTER2.
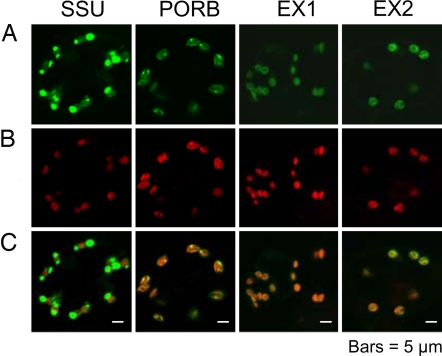
During an extensive second-site-mutant screen of the flu mutant, 15 different allelic lines of executer1 (ex1) were identified. In three of these mutant lines, the mutations led to an amino acid exchange (12). These amino acid residues are conserved among all EXECUTER1 proteins of higher plants for which sequence data are available (12). A second EXECUTER1-like gene was found in Arabidopsis that was dubbed EXECUTER2 and that was considered to be a candidate gene for a second putative signal component involved in 1O2-dependent signaling. The predicted overall amino acid sequence identity between EXECUTER1 and EXECUTER2 is 38%, but the sequence identity increases to 42%, if only sequences of mature proteins without the signal sequences are compared (Fig. 1). EXECUTER1 and EXECUTER2 of Arabidopsis are closely related to the corresponding proteins of the monocotyledonean plant rice (Fig. 1). In particular the C termini of the EXECUTER proteins are highly conserved (Fig. 1). The three highly conserved amino acid residues of EXECUTER1 that seem to be essential for its activity are also conserved in all EXECUTER2 proteins of higher plants for which sequence data are available (Fig. 1; unpublished data). The ORF of EXECUTER2 predicts a protein of 652 aa with a molecular mass of 72 kDa. Like EXECUTER1, it is unrelated to known proteins, except that its N-terminal part resembles import signal sequences of nuclear-encoded plastid proteins. This prediction was confirmed experimentally by expressing EXECUTER1- and EXECUTER2-GFP fusion proteins in stably transformed Arabidopsis plants and determining their intracellular localization under the confocal microscope (Fig. 2). As controls also plants expressing the small subunit (SSU) of the ribulose-1,5-bisphosphate carboxylase-GFP fusion protein and the NADPH-protochlorophyllide oxidoreductase(POR)B-GFP fusion protein were analyzed (Fig. 2). The former accumulates within the stroma of plastids, whereas PORB is part of the chloroplast membranes (13, 14). Both EXECUTER1 and EXECUTER2 accumulate within chloroplasts and seem to be associated with thylakoid membranes (Fig. 2).
Fig. 1.
Multiple alignments of deduced amino acid sequences of full-length cDNAs of EXECUTER1 and EXECUTER2 from Arabidopsis and rice. The three highly conserved amino acid residues of EXECUTER1 that were identified in a previous suppressor mutant screen of flu (12) are indicated by arrow heads. The amino acid sequences were aligned by using the ClustalW program. Gaps, which were introduced to maximize the alignment, are indicated by dashes. AtEX1(NP_567929) and AtEX2(NP_564287) from A. thaliana, OsEX1(AAL59023) and OsEX2 (BAD44852) from rice.
Fig. 2.
Intracellular accumulation of GFP fusions with the small subunit of ribulose-1,5-bisphosphate carboxylase (SSU), the NADPH-Pchlide oxidoreductase B (PORB), EXECUTER1 (EX1), and EXECUTER2 (EX2) in cotyledons of transgenic seedlings grown for 5 days under continuous light. The green fluorescence of GFP fusion proteins (A) and the red fluorescence of chlorophyll (B) were monitored separately by using a confocal laser scanning microscope, and the the two fluorescence images were merged (C).
Functional Characterization of EXECUTER2.
During the second-site mutant screen of flu a large number of allelic ex1 mutant lines, but no executer2 (ex2) mutants have been found (12). These results suggest that the EXECUTER2 protein is not essential for mediating the visible stress responses that have been used for the selection of second-site mutants (12). However, this conclusion does not preclude the possibility that EXECUTER2 is involved in mediating other 1O2-dependent stress responses. These predictions were tested experimentally by first identifying an EXECUTER2 mutant line and crossing it with flu and then studying the effect of EXECUTER2 inactivation on 1O2-mediated stress responses in the flu background.
We have identified an Arabidopsis T-DNA [portion of the Ti (tumor-inducing) plasmid that is transferred to plant cells] insertion line from the SALK collection with a predicted insertion of the T-DNA in the EXECUTER2 gene. The genetic background of this line was Columbia (Col-0). Because the ex1 mutation had been found originally in Ler we searched for and identified a Col-0 line with the insertion of the T-DNA also predicted to be in the EXECUTER1 gene. This prediction could be confirmed by PCR (data not shown). Both T-DNA-insertion lines were crossed with each other and a flu Col-0 line. Mature plants of the resulting ex1/flu, ex2/flu and ex1/ex2/flu mutant lines, flu, and wild type, all in Col-0, were subjected to the same dark/light shift experiment used previously to characterize the flu and ex1 mutations in the Ler lines (12).
Mutant and wild-type plants were grown under continuous light until they reached the rosette leaf stage and were ready to bolt. Plants were then shifted from continuous light to a 16 h light/8 h dark program for the next 30 days. Once they were transferred to the long day conditions, flu and ex2/flu plants stopped growing, whereas ex1/flu and ex1/ex2/flu plants continued to grow similar to wild type except that their growth was slightly reduced and their final height was ≈80% of that of wild type. Under continuous light all five lines grew equally well and finally reached the same height (Fig. 3). When mature plants grown under continuous light were shifted to the dark for 8 h free Pchlide accumulated in all four mutant lines to similar levels in rosette leaves and were 3- to 4-fold higher than in wild-type controls.
Fig. 3.
1O2-mediated growth inhibition of mature ex1/flu, ex2/flu, ex1/ex2/flu, flu, and wild-type (wt) plants. Plants were grown for 21 days under continuous light until they were ready to bolt. Plants were then either shifted to a 16 h light/8 h dark program (A) or kept under continuous light (B), and the elongation of the inflorescence was followed over the next 30 days. Long-day conditions were used instead of short-day conditions to avoid the overaccumulation of excess amounts of Pchlide during an extended dark period. Under these light conditions, toxic effects of 1O2 could be minimized. In contrast to Ler plants used previously (9, 12), the onset of bolting of Col-0 varied greatly between different plants. Growth curves of individual plants were corrected for these differences. Each value represents the average growth measurements of 10 different plants. (C) The accumulation of Pchlide in 21-day-old plants grown under continuous light and transferred to the dark for 8 h. Total Pchlide was extracted from aerial parts of single plants and analyzed by HPLC. For each genotype, seven independent Pchlide measurements are shown.
Collectively, these results demonstrate that in the Col-0 background the effect of the flu mutation is similar to that in Ler (9, 12). Furthermore, also in Col-0 the ex1 mutation in the flu background suppresses singlet oxygen-mediated growth inhibition of mature plants, whereas inactivation of the EXECUTER2 gene of the flu mutant has only a minor effect on this 1O2-mediated stress response, as one would expect based on our previous failure to isolate ex2/flu double mutants during an extensive second-site mutant screen of flu (12). Mature ex1/ex2 mutant plants without the flu mutation are phenotypically similar to wild type (data not shown).
The Effects of EXECUTER1 and EXECUTER2 Inactivation on 1O2-Dependent Signaling of Nuclear Gene Expression.
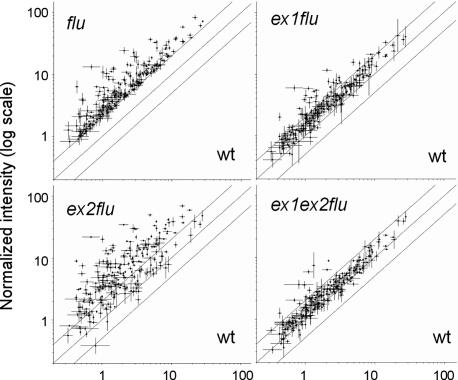
The impact of the ex1 and ex2 mutations on rapid 1O2-mediated changes in nuclear gene expression was analyzed by growing plants for 3 weeks under continuous light, until they were ready to bolt. Plants at the rosette leaf stage were transferred to the dark for 8 h and reexposed to light for 30 min. Total RNA was extracted from the leaves and was first transcribed into cDNAs and then into biotinylated complementary RNAs that were hybridized to Affymetrix gene chips. Genes with a 2-fold or greater transcript level than the control were considered to be significantly up-regulated. After 30 min of reillumination, a total of 245 genes had been up-regulated in flu relative to wild type (Figs. 4 and 5). This number in ecotype Col-0 was lower than that in flu Ler reported earlier (9). Inactivation of EXECUTER1 led to a dramatic drop in the number of up-regulated genes from 245 in flu down to 54 in ex1/flu (Fig. 5). This suppressive effect of EXECUTER1 inactivation in flu can also be seen by comparing scatter plots of nuclear transcripts of genes significantly up-regulated in flu and suppressed in ex1/flu (Fig. 4). EXECUTER1 seems to play a major role during the up-regulation of nuclear genes in the flu mutant, but its absence in ex1/flu double mutants does not completely eliminate activation of 1O2-responsive genes. Therefore, additional components must be implicated with the 1O2-induced transduction of signals from the plastid to the nucleus.
Fig. 4.
The impact of EXECUTER1 and EXECUTER2 mutations on the up-regulation of 1O2-responsive nuclear genes in the flu mutant. Plants (Col-0) were grown for 21 days under continuous light, shifted to the dark for 8 h, and reexposed to light for 30 min. Global changes in transcript levels were determined by using Affymetrix gene chips. Among 13,600 genes that were selected as present in all replicas, 245 were up-regulated at least 2-fold in flu relative to wild type. Transcript levels of these selected genes are shown in scatter plots of flu versus wild type, ex1/flu versus wild type, ex2/flu versus wild type, and ex1/ex2/flu versus wild type. The individual dots shown on the scatter plots were derived as average expression values from both replicate experiments.
Fig. 5.
The impact of EXECUTER1 and EXECUTER2 mutations on 1O2-mediated changes in nuclear gene expression. The relationships of four selected groups of genes up-regulated at least 2-fold in flu versus wild type, ex1/flu versus wild type, ex2/flu versus wild type, and ex1/ex2/flu versus wild type were analyzed by using a Venn diagram. A subset of five genes up-regulated only in flu and ex1/ex2/flu relative to wild type is not shown in the Venn diagram, but has been included in SI Data Set 1. Plants were grown and treated as described under Fig. 4.
EXECUTER2 may play a supplementary role during 1O2-mediated signaling that may account for the residual activation of 1O2-responsive genes in ex1/flu after a dark-to-light shift. This proposition was tested experimentally first by analyzing in ex2/flu the transcript profiles of those genes that in flu were at least 2-fold up-regulated relative to wild type. The scatter plot analysis of these transcripts in ex2/flu revealed that inactivation of EXECUTER2 modified drastically the up-regulation of 1O2-responsive genes in the flu mutant by further enhancing or reducing the transcript levels of these genes (Fig. 4). In a subsequent step, additional genes were included in this analysis that were up-regulated in the ex1/flu, ex2/flu and ex1/ex2/flu mutant lines relative to wild type [Fig. 5 and supporting information (SI) Data Set 1]. The majority of 1O2-up-regulated genes in the flu mutant are found in a cluster of 178 genes that are up-regulated both in flu as well as in ex2/flu (Fig. 5, groups A, I, and M). Unexpectedly, in ex2/flu the up-regulation of a larger part of these genes is significantly higher than in flu (Fig. 4). Half of the genes with an assigned function have been associated with signaling, gene transcription and stress responses. Among the genes predicted to encode transcription factors and DNA-binding proteins, nine belong to the large gene family of WRKY transcription factors that have been associated with various disorders such as stress, aging, senescence and diseases (15). The enhanced expression of 1O2-responsive genes caused by the inactivation of EXECUTER2 is also reflected in the appearance of additional 1O2-responsive genes that are significantly up-regulated in ex2/flu but not in flu (Fig. 5, groups L and G). Approximately half of these genes are of unknown function. Similar to the gene groups A, I, and M, also in groups L and G >50% of the remaining genes encode proteins predicted to be involved in transcription, signaling or stress-related responses (SI Data Set 1).
Inactivation of EXECUTER2 does not only accelerate the expression of a large number of 1O2-responsive genes, but at the same time also evokes the down-regulation of a subset of 62 1O2-responsive genes (Fig. 5, groups E, B, and J). Up-regulation of 59 of these genes in response to 1O2 generated in chloroplasts of the flu mutant depends on the combined activities of EXECUTER1 and EXECUTER2 (Fig. 5, group E). In ex1/flu and ex2/flu, but also in the ex1/ex2/flu triple mutant the 1O2-induced enhanced expression of these genes is suppressed. Several of these genes have been associated with various stress-related responses such as two trehalose 6-phosphate synthetase genes which have been implicated in conferring desiccation tolerance to plants (16, 17). Collectively, these results reemphasize a key role of EXECUTER1 in stimulating the up-regulation of a larger number of nuclear genes that comprise the majority of 1O2-responsive genes in the flu mutant. At the same time, they reveal a striking regulatory role of EXECUTER2 that seems to attenuate and antagonize the activity of EXECUTER1. However, EXECUTER2 alone in the absence of active EXECUTER1 has only a limited effect on the expression of 1O2-responsive genes. Because of the reciprocal activities of the two EXECUTER proteins in the flu mutant that impact each other during the 1O2-induced transfer from the plastid to the nucleus, it was of interest to see whether inactivation of both these proteins in the ex1/ex2/flu triple mutant would completely abrogate the singlet oxygen-mediated up-regulation of nuclear genes. Almost all of the transcripts that in flu had been up-regulated at least twofold remained in the triple mutant below the 2-fold threshold value, but were still slightly higher than in the wild-type control. Six of the 1O2-responsive genes were significantly up-regulated only in the triple mutant relative to wild type (Fig. 5, group H). Two of these genes encode proteins of unknown function. One of the four genes with an assigned function is predicted to encode an auxin-responsive transcription factor. At the same time two of seven genes that are significantly up-regulated in ex1/flu are also involved in auxin-dependent responses (Fig. 5, group F; SI Data Set 1).
Among the genes that had been shown by the Affymetrix chip analysis to be induced stronger in ex2/flu than in flu, four were selected and changes in their transcript levels were quantified independently by using real-time PCR to test the reliability of the Affymetrix chip analysis (Fig. 6). The expression of genes that encode the WRKY33 (At2g38470) and WRKY46 (At2g46400) transcription factors (15), a disease resistance protein (At1g66090) and the 1-amino-cyclopropane-1 carboxylic acid (ACC) synthase 6 (At1g11280) (18) were up-regulated in flu during the first 30 min of reillumination. For each gene the transcript level was 2- to 3-fold higher in ex2/flu than in flu, whereas in ex1/flu and the triple mutant these levels were down-regulated and similar to those of wild type.
Fig. 6.
Activation of four 1O2-responsive genes in flu and ex2/flu and their suppression in ex1/flu and ex1/ex2/flu mutant plants. Plants were grown for 21 days under continuous light, transferred to the dark for 8 h, and in some cases reexposed to light for 30 min. Transcript levels of WRKY33 (At2g38470) (A), WRKY46 (At2g46400) (B), a putative disease resistance gene (At1g66090) (C), and the gene encoding the 1-amino-cyclopropane-1 carboxylic acid (ACC) synthase 6 (At1g11280) (D) were determined by Real-Time PCR. The results represent average values of measurements from three independent experiments ± SE. RNA was extracted at the end of the dark period (D) or after 30 min of reillumination (D → L).
Discussion
In our present work, we have used the conditional flu mutant to characterize the physiological role of 1O2 that is generated within the plastid compartment after a dark-to-light shift. Shortly after the release of 1O2 drastic changes in nuclear gene expression occur that reveal a rapid transfer of signals from the plastid to the nucleus. Because 1O2 is very unstable and unlikely to leave the plastid compartment (19, 20), its physiological impact has been attributed to the generation of more stable second messengers within the plastid that are assumed to activate a signaling pathway and control the expression of a large number of nuclear genes (9). EXECUTER1 seems to play a key role during the transfer of signals from the plastid to the nucleus. Its biological activity, however, depends on its interaction with a second closely related protein, EXECUTER2. Even though it is not known yet whether EXECUTER1 and EXECUTER2 physically interact with each other, such a direct contact would be in line with some of the results of our present work. The two proteins localize in chloroplasts and seem to be both associated with thylakoid membranes. Upon inactivation of EXECUTER2 in the flu mutant, additional 1O2-responsive genes emerge and genes that were already up-regulated in flu are either further stimulated or down-regulated. In the absence of EXECUTER1, EXECUTER2 has only a relatively minor effect on the expression of 1O2-responsive genes (see e.g., Fig. 4). Thus, the primary function of EXECUTER2 seems to be that of a modulator attenuating and controlling the activity of EXECUTER1. Inactivation of EXECUTER1 greatly reduces but does not completely eliminate the up-regulation of nuclear 1O2-responsive genes. Only when both EXECUTER proteins are inactive is the up-regulation of the vast majority of 1O2-responsive genes abolished.
The EXECUTER1- and EXECUTER2-dependent signaling in the flu mutant bears a striking resemblance to retrograde signaling that has been shown to play a central role in controlling gene expression in the nucleus and the plastid (21, 22). Chloroplast proteins are encoded by both nuclear and plastid genomes (23). Because of this separation of the genetic information, the expression of these two genomes needs to be coordinated. It is well established that the development and activity of chloroplasts depend on the synthesis and import of a large number of nuclear-encoded plastid proteins (24). On the other hand, the expression of at least some of the nuclear genes depends on the functional state of the plastid by means of a process known as retrograde signaling (25–27).
Initially the biological impact of plastid-derived signals had been considered to be confined to the fine-tuning and coordination of nuclear and chloroplast gene activities that are required for the optimization and protection of chloroplast-specific functions such as e.g., photosynthesis (21, 22, 25). The results of our work demonstrate that the primary function of singlet oxygen in the flu mutant does not seem to be the control of chloroplast performance but the activation of a stress-related signaling cascade that encompasses numerous signaling pathways known to be activated by pathogen attack, wounding, light and drought stress (28–30).
Less than 15% of the 1O2-responsive genes of the flu mutant are predicted to encode plastid proteins and none of these genes can be linked to photosynthesis or the control of chloroplast development, whereas a large fraction of 1O2-responsive genes are known to be involved in different stress responses. The 1O2-activated cell death program and growth inhibition resemble stress-related resistance strategies of higher plants (31, 32). These 1O2-dependent stress responses of the flu mutant were suppressed after the inactivation of EXECUTER1 and EXECUTER2. Both the generation of 1O2 within plastids and the plastid-specific localization of the EXECUTER1 and 2 proteins reiterate the importance of chloroplasts as a major source of stress-related signals.
The activation of a suicidal program in seedlings and the block of growth in mature plants of flu has not been reported to occur in wild-type plants even under conditions that would be expected to stimulate the release of 1O2. This apparent difference between flu and wild type may question the physiological relevance of 1O2-mediated stress responses of the flu mutant. EXECUTER1 and EXECUTER2 are highly conserved among all higher plants for which sequence data are available. This conservation is consistent with EXECUTER1 and EXECUTER2 being involved in processes that are both beneficial and common to higher plants. The overaccumulation of the photosensitizer Pchlide and the sudden shift from the dark to the light that in the flu mutant evokes the instantaneous release of 1O2 does normally not occur in wild-type plants. Conditions to which wild-type plants are genetically adapted and that endorse the enhanced production of 1O2 would thus be expected to induce the release of modulating factors that control and subdue the extreme 1O2-mediated stress responses as seen in flu. Two such modulating activities have recently been identified. Various stress conditions may lead to the hyperreduction of the photosynthetic electron transfer chain that blocks electron transfer by PSII and enhances the production of 1O2 (33, 34). Plants may use additional electron sinks to maintain the acceptor site of PSII in a partially oxidized state (8). One of these additional electron acceptors is molecular oxygen. It can be reduced by PSI to superoxide that is rapidly converted to hydrogen peroxide (35). Hydrogen peroxide has been shown recently to antagonize the biological activity of 1O2 and to suppress 1O2-mediated cell death and growth inhibition (36). Another modulation of 1O2-dependent stress responses has been attributed to acclimation activated by minor stress conditions that precede the release of 1O2 (M. Würsch and K.A., unpublished results). Therefore, EXECUTER1- and EXECUTER2-dependent signaling of stress responses in wild-type plants seems to form an integral part of a complex signaling network and is subject to the control by various modulators that weaken the extreme consequences of this signaling as seen in the flu mutant. As shown in the present work, the flu mutant offers a way of how to penetrate and dissect this complexity and identify individual signaling pathways.
Methods
Plant Material.
The EX1 (At4g33630) T-DNA insertion line SALK_002088 and EX2 (At1g27510) T-DNA insertion line SALK_012127 were obtained from the European Arabidopsis Stock Centre (NASC). Homozygous mutant lines were identified by PCR analysis by using T-DNA-, EX1- and EX2-specific primers. Both T-DNA-lines were crossed with a flu Col-0 line that had been obtained by 5 backcrosses of flu1-1 in Landsberg erecta with wild-type Columbia. The ex1/flu and ex2/flu mutant lines were crossed, and within the segregating F2 population triple mutants were identified by PCR-based genotyping. For the cultivation of mature plants, seeds of wild type, flu, ex1/flu, ex2/flu, and ex1/ex2/flu, all in Col-0 ecotype, were sown on soil and plants were grown under continuous light (100 μmol·m−2·s−1).
Extraction and Measurement of Protochlorophyllide.
Pchlide was extracted separately from seven biological samples of each of the 5 genotypes (wild type, flu, ex1/flu, ex2/flu, and ex1/ex2/flu) growing under continuous light (100 μmol·m−2·s−1) for 21 days and then transferred to the dark for 8 h. After the end of dark periods, samples were harvested and homogenized with liquid nitrogen under green safety light. About 0.1g of the powdered samples were suspended in 1 ml of cold 90% acetone, and centrifuged for 5 min at 9,300 × g. The supernatants were used to determine the level of Pchlide by HPLC according to Kim and Apel (13).
RNA Extraction and Real-Time PCR.
Total RNA was extracted by using an RNeasy plant mini kit (Qiagen, Hilden, Germany) and quantified spectrophotometrically at 260 nm. For the real-time PCR, RNAs were treated with RQ1 RNase-Free DNase (Promega, Madison, WI) and reverse-transcribed by using oligo(dT)15 primer (Promega) and and Improm II reverse transcriptase (Promega) according to the manufacturer's recommendations. Real-time PCR was performed with equal amounts of cDNAs by using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA), a SYBR Green PCR kit from Applied Biosystems, and gene-specific primers. Relative mRNA abundance was calculated by using the comparative delta-Ct method and normalized to the ACT2 (At3g18780) gene levels. The sequences of the primers for the selected genes are: At2g38470, GAAACAAATGGTGGGAATGG and TGTCGTGTGATGCTCTCTCC; At2g46400, GATCCTTAAGCGAAGCCTTG and TCGATGCGTGCATCTGTAAT; At4g11280, GACGAGTTTATCCGCGAGAG and ACACGCCATAGTTCGGTTTC; At1g66090, AACCGGAGTACACGTCCAAG and CGGAGATCCCAACGATCTTA.
Microarray Hybridization and Analysis.
Two individual biological replicates, each containing material of five mature plants of wild type, flu, ex1/flu, ex2/flu, and ex1/ex2/flu, respectively, were used for the microarray analysis. Plants were germinated on soil and kept under continuous light until the beginning of bolting and then transferred to the dark for 8 h. Dark-incubated mature plants were reilluminated for 30 min and subsequently harvested for RNA extraction. Total RNA was prepared as described in SI Materials and Methods.
Growth Measurements.
Growth of the primary stem was determined by measuring its length daily for 30 days with wild type, flu, ex1/flu, ex2/flu, and ex1/ex2/flu growing under continuous light (100 μmol·m−2·s−1) or under long day conditions (16 h light/8 h dark).
Construction and Detection of the GFP Fusion Proteins in Vivo.
A modified pCAMBIA 3300 binary vector containing the CaMV 35S promoter, a NcoI cloning site, the EGFP-sequence and the terminal polyadenylation site was used as a basis for all subsequent constructions (13). For the in vivo localization of the fusion protein, full-length EXECUTER1 and EXECUTER2 without their stop codons were amplified from the cDNA of Arabidopsis thaliana (Col-0) and subcloned between the promoter and EGFP of the modified pCAMBIA 3300 vector. To amplify this plasmid, competent Escherichia coli cells (DH5α) were used. Competent cells of Agrobacterium tumefaciens C58 were transformed with the plasmid and then used for stable in planta transformation of Arabidopsis Col-0. The primary transgenic plants were selected on MS agar plates containing phosphinothricin (25 mg/l) and transferred to soil to harvest seeds. The green fluorescence of GFP and the red fluorescence of chlorophyll were monitored by using a Confocal Laser Scanning Microscope (TCS-NT; Leica Microsystems, Heidelberg, Germany) according to Kim and Apel (13).
Other Methods.
For homology searches and protein structure predictions, National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/BLAST/) and ExPASy Molecular Biology Server (www.expasy.ch) were used.
For multiple sequence alignment, ClustalW (www.ebi.ac.uk/clustalw/) and Boxshade 3.21 (www.ch.embnet.org/software/BOX_form.html) were used.
Supplementary Material
Acknowledgments
We thank André Imboden for taking care of plants and measuring growth, Mena Nater for doing numerous crosses and identifying mutant lines, Jean-Charles Isner for help with the HPLC measurements, and Dr. Dieter Rubli for photographs. We thank members of our group, in particular Drs. Rasa Meskauskiene and Christophe Laloi for critical comments. We acknowledge the editorial work of Ursula Baldenweg. This work was supported by grants from the Swiss National Science Foundation (NSF), the Functional Genomic Center Zurich (FGCZ), and the ETH-Zurich.
Abbreviations
- ROS
reactive oxygen species
- Pchlide
protochlorophyllide
- T-DNA
portion of the Ti (tumor-inducing) plasmid that is transferred to plant cells.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702061104/DC1.
References
- 1.Elstner EF, Osswald W. Proc R Soc Edinburgh. 1994;102:131–154. [Google Scholar]
- 2.Neill S, Desikan R, Hancock J. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 3.Apel K, Hirt H. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 4.Torres MA, Dangl JL, Jones JD. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foreman J, Demidchick V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B. Plant Cell. 2006;18:1052–1066. doi: 10.1105/tpc.105.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niyogi KK. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 9.op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim CH, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, et al. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp RO, Apel K. Proc Natl Acad Sci USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollnick K. Adv Photochem. 1968;6:1–122. [Google Scholar]
- 12.Wagner D, Przybyla D, op den Camp RG, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Apel K. Plant Cell. 2004;16:88–98. doi: 10.1105/tpc.015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths WT. Biochemistry. 1978;174:681–692. doi: 10.1042/bj1740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. Trends Plants Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 16.Müller J, Boller T, Wiemken A. Plant Sci. 1995;112:1–9. [Google Scholar]
- 17.Leyman B, Van Dijck P, Thevelein JM. Trends Plants Sci. 2001;6:510–513. doi: 10.1016/s1360-1385(01)02125-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu YD, Zhang SQ. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sies H, Menck CFM. Mutat Res. 1992;275:367–375. doi: 10.1016/0921-8734(92)90039-r. [DOI] [PubMed] [Google Scholar]
- 20.Gorman AA, Rodgers MAJ. Photochem Photobiol B. 1992;14:159–176. doi: 10.1016/1011-1344(92)85095-c. [DOI] [PubMed] [Google Scholar]
- 21.Beck CF. Planta. 2005;222:743–756. doi: 10.1007/s00425-005-0021-2. [DOI] [PubMed] [Google Scholar]
- 22.Rodermel S. Trends Plant Sci. 2001;6:471–478. doi: 10.1016/s1360-1385(01)02085-4. [DOI] [PubMed] [Google Scholar]
- 23.Bogorad L. Science. 1975;188:891–898. doi: 10.1126/science.1138359. [DOI] [PubMed] [Google Scholar]
- 24.Kirk JTO, Tilney-Bassett RAE. The Plastids: Their Chemistry, Structure, Growth and Inheritance. 2nd Ed. New York: Elsevier; 1978. [Google Scholar]
- 25.Mayfield SP, Taylor WC. Eur J Biochem. 1984;144:79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- 26.Kropat J, Oster U, Rudiger W, Beck CF. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strand A, Asami T, Alonso J, Ecker JR, Chory J. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 28.Danon A, Miersch O, Felix G, op den Camp RG, Apel K. Plant J. 2005;41:68–80. doi: 10.1111/j.1365-313X.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Ochsenbein C, Przybyla D, Danon A, Landgraf F, Göbel C, Imboden A, Feussner I, Apel K. Plant J. 2006;47:445–456. doi: 10.1111/j.1365-313X.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 30.Danon A, Sanchez Coll NS, Apel K. Proc Natl Acad USA. 2006;103:17036–17041. doi: 10.1073/pnas.0608139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netting AG. J Exp Bot. 2000;51:147–158. doi: 10.1093/jexbot/51.343.147. [DOI] [PubMed] [Google Scholar]
- 32.Bray EA, Bailey-Serres J, Weretilnyk E. In: Biochemistry and Molecular Biology of Plants. Buchanan BB, Gruissem W, Jones RL, editors. Rockville, MD: Am Soc Plant Physiol; 2002. pp. 1158–1203. [Google Scholar]
- 33.Hideg E, Barta C, Kalai T, Vass I, Hideg K, Asada K. Plant Cell Physiol. 2002;43:1154–1164. doi: 10.1093/pcp/pcf145. [DOI] [PubMed] [Google Scholar]
- 34.Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. J Exp Bot. 2002;53:1249–1254. [PubMed] [Google Scholar]
- 35.Asada K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 36.Laloi C, Stachowiak M, Pers-Kamczyk E, Warzych E, Murgia I, Apel K. Proc Natl Acad Sci USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.